Abstract
Purpose
Myasthenia gravis (MG) is a rare but life-threatening complication of immune-checkpoint inhibitor (ICI) therapy and often co-presents with myositis and myocarditis. Previous case series of ICI-related MG have reported high mortality rates. We present a series of ten patients from a tertiary oncology centre outlining outcomes of an early multi-modal immunosuppression strategy.
Methods
We reviewed The Christie Hospital database of immunotherapy-related toxicity from 2017 to 2020. Symptom severity was assessed using the Myasthenia Gravis Foundation of America (MGFA) classification.
Results
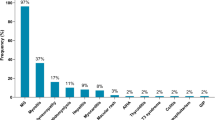
Ten patients with ICI-related MG were identified. All patients presented following 1 (n = 4) or 2 (n = 6) cycles of ICI. Symptom progression was rapid with a median of 3 days from onset of symptoms to admission. Concomitant myositis and myocarditis were observed in nine patients. AChR or MuSK autoantibodies were positive in six patients. All patients received urgent treatment with intravenous methylprednisolone (IVMP) and eight received intravenous immunoglobulin (IVIG). A single patient died from myasthenia-related symptoms; the remaining 9 patients were successfully discharged.
Conclusion
In our cohort, we demonstrate good outcomes associated with early intensive immunosuppressive treatment with IVIG and IVMP. An agreed national treatment protocol or clinical discussion forum would be beneficial.

Similar content being viewed by others
References
Johnson DB, Manouchehri A, Haugh AM et al (2019) Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunotherapy Cancer 7:134
Cooksley T, Gupta A, Al-Sayed T, Lorigan P (2020) Emergency presentations in patients treated with immune checkpoint inhibitors. Eur J Cancer 130:193–197
Mikami T, Liaw B, Asada M et al (2021) Neuroimmunological adverse events associated with immune checkpoint inhibitor: a retrospective, pharmacovigilance study using FAERS database. J Neuro-Oncol 152(1):135–144
Pathak R, Katel A, Massarelli E et al (2021) Immune checkpoint inhibitor-induced myocarditis with myositis/myasthenia gravis overlap syndrome: a systematic review of cases. Oncologist 26(12):1052–1061
Safa H, Johnson DH, Trinh VA et al (2019) Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer 7(1):319
Suzuki S, Ishikawa N, Konoeda F et al (2017) Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 89(11):1127–1134
Suarez-Almazor ME, Pundole X et al (2020) Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of immune-mediated cardiovascular, rheumatic, and renal toxicities from checkpoint inhibitors. Support Care Cancer 28(12):6159–6173
Narayanaswami P, Sanders DB, Wolfe G et al (2021) International consensus guidance for management of myasthenia gravis: 2020 update. Neurology 96(3):114–122
Brahmer JR, Abu-Sbeih H, Ascierto PA et al (2021) Society for Immunotherapy of Cancer (SITC) Clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 9:e002435
Author information
Authors and Affiliations
Contributions
JW, JL, MR, PL and TC conceived the implementation of the project and wrote the main manuscript text and prepared the figures. TK, MC and RK collected data, assisted in manuscript and figure preparation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is an observational study with no ethical approval required. Local institutional board number: 3180.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weaver, J.M., Dodd, K., Knight, T. et al. Improved outcomes with early immunosuppression in patients with immune-checkpoint inhibitor induced myasthenia gravis, myocarditis and myositis: a case series. Support Care Cancer 31, 518 (2023). https://doi.org/10.1007/s00520-023-07987-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07987-x