Abstract
Purpose
Cutaneous lymphomas (CLs) are a group of rare, potentially disfiguring and disabling cancers that can have a significant impact on quality of life (QoL). While previous studies have shown that mycosis fungoides (MF) and Sézary syndrome (SS) impair QoL, the effect of other types of CL on QoL has not been evaluated.
Objective
To determine the impact of disease on QoL in all CL patients and to assess how QoL between the CL sub-types varies by demographic and clinical factors.
Methods
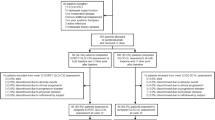
The Cutaneous Lymphoma Distress Questionnaire (CL-DQ) was used to assess QoL. All CL patients seen in a multidisciplinary CL clinic were screened for eligibility. Questionnaire responses were collected over a 22-month period between 2017 and 2019. A cross-sectional analysis of CL-DQ scores from an initial visit was performed to determine the effect of disease on QoL across CL sub-types and the potential impact of patient demographics, CL sub-type, and type of treatment.
Results
The study population consisted of 151 patients presenting with distinct types of cutaneous B- and T-cell lymphomas. Notable across the study population were the findings of frustration (44%), worry about progress/spread (43%), itching/pruritus (32%), and embarrassment/shame (28%). QoL was found to be most negatively affected in SS patients, females, younger patients, Black patients, and those with advanced stages of MF/SS.
Conclusions
Impairment of QoL due to CL correlates with gender, age, race/ethnicity, and stage of MF/SS. While the negative impact on QoL is most pronounced in SS patients, other CL sub-types also affect QoL and impact psychosocial distress. Our findings highlight the need for QoL assessment in all CL patients and further examination of disparities noted across demographic groups.


Similar content being viewed by others
Data Availability
De-identified data can be made available upon request.
Code availability
Upon request.
References
Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019.
Demierre MF, Gan S, Jones J, Miller DR (2006) Significant impact of cutaneous T-cell lymphoma on patients’ quality of life: results of a 2005 National Cutaneous Lymphoma Foundation Survey. Cancer 107(10):2504–2511
Demierre MF, Tien A, Miller D (2005) Health-related quality-of-life assessment in patients with cutaneous T-cell lymphoma. Arch Dermatol 141(3):325–330
Holahan HM, Farah RS, Fitz S, Mott SL, Ferguson NN, McKillip J et al (2018) Health-related quality of life in patients with cutaneous T-cell lymphoma? Int J Dermatol 57(11):1314–1319
Molloy K, Jonak C, Woei AJF, Guenova E, Busschots AM, Bervoets A, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sezary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. The British journal of dermatology. 2019.
Sampogna F, Frontani M, Baliva G, Lombardo GA, Alvetreti G, Di Pietro C et al (2009) Quality of life and psychological distress in patients with cutaneous lymphoma. Br J Dermatol 160(4):815–822
Wright A, Wijeratne A, Hung T, Gao W, Whittaker S, Morris S et al (2013) Prevalence and severity of pruritus and quality of life in patients with cutaneous T-cell lymphoma. J Pain Symptom Manage 45(1):114–119
Jonak C, Porkert S, Oerlemans S, Papadavid E, Molloy K, Lehner-Baumgartner E et al (2019) Health-related quality of life in cutaneous lymphomas: past, present and future. Acta Derm Venereol 99(7):640–646
Posada-Cano AM, Valencia-Ocampo OJ, Velásquez-Lopera MM (2019) Assessment of the quality of life in patients with cutaneous lymphoma. Dermatol Rev Mex 63(1):26–39
Chren MM, Lasek RJ, Sahay AP, Sands LP (2001) Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg 5(2):105–110
Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ (1997) Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol 133(11):1433–1440
Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R et al (2007) Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 110(6):1713–1722
Johnson DR, Creech JC (1983) Ordinal measures in multiple indicator models: a simulation study of categorization error. Am Sociol Rev 48(3):398–407
Norman G (2010) Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract 15(5):625–632
Sullivan GM, Artino AR Jr (2013) Analyzing and interpreting data from likert-type scales. J Grad Med Educ 5(4):541–542
Zumbo BD, Zimmerman DW (1993) Is the selection of statistical methods governed by level of measurement? Can Psychol 34(4):390–400
Bradford PT, Devesa SS, Anderson WF, Toro JR (2009) Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood 113(21):5064–5073
Geller S, Lebowitz E, Pulitzer MP, Horwitz SM, Moskowitz AJ, Dusza S et al (2020) Outcomes and prognostic factors in African American and black patients with mycosis fungoides/Sezary syndrome: retrospective analysis of 157 patients from a referral cancer center. J Am Acad Dermatol 83(2):430–439
Imam MH, Shenoy PJ, Flowers CR, Phillips A, Lechowicz MJ (2013) Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma 54(4):752–759
Nath SK, Yu JB, Wilson LD (2014) Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk 14(5):419–423
Su C, Nguyen KA, Bai HX, Cao Y, Tao YG, Xiao R, et al. Racial disparity in mycosis fungoides: An analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77(3):497-+.
Susman E (2016) Sézary syndrome discriminates among ethnicities. Oncology Times 38(11):20
Weinstock MA, Reynes JF (1999) The changing survival of patients with mycosis fungoides: a population-based assessment of trends in the United States. Cancer 85(1):208–212
Samuel CA, Pinheiro LC, Reeder-Hayes KE, Walker JS, Corbie-Smith G, Fashaw SA et al (2016) To be young, Black, and living with breast cancer: a systematic review of health-related quality of life in young Black breast cancer survivors. Breast Cancer Res Treat 160(1):1–15
Ye J, Shim R, Garrett SL, Daniels E (2012) Health-related quality of life in elderly black and white patients with cancer: results from Medicare managed care population. Ethn Dis 22(3):302–307
Lubeck DP, Kim H, Grossfeld G, Ray P, Penson DF, Flanders SC et al (2001) Health related quality of life differences between black and white men with prostate cancer: data from the cancer of the prostate strategic urologic research endeavor. J Urol 166(6):2281–2285
Ashing-Giwa K, Ganz PA, Petersen L (1999) Quality of life of African-American and white long term breast carcinoma survivors. Cancer 85(2):418–426
Ramsey SD, Zeliadt SB, Hall IJ, Ekwueme DU, Penson DF (2007) On the importance of race, socioeconomic status and comorbidity when evaluating quality of life in men with prostate cancer. J Urol 177(6):1992–1999
Ayanian JZ, Zaslavsky AM, Guadagnoli E, Fuchs CS, Yost KJ, Creech CM et al (2005) Patients’ perceptions of quality of care for colorectal cancer by race, ethnicity, and language. J Clin Oncol Off J Am Soc Clin Oncol 23(27):6576–6586
Kale HP, Carroll NV (2016) Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer 122(8):283–289
Krupski TL, Fink A, Kwan L, Maliski S, Connor SE, Clerkin B et al (2005) Health-related quality-of-life in low-income, uninsured men with prostate cancer. J Health Care Poor Underserved 16(2):375–390
Porkert S, Lehner-Baumgartner E, Valencak J, Knobler R, Riedl E, Jonak C (2018) Patients’ illness perception as a tool to improve individual disease management in primary cutaneous lymphomas. Acta Derm Venereol 98(2):240–245
Acknowledgements
We would like to thank our patients that participated in this study, and all of our clinical staff for their assistance and tireless efforts to improve the lives of our patients.
Funding
This research included work performed by the Biostatistics and Mathematical Oncology Core supported through NIH/NCI Cancer Center Support Grant (P30CA033572) to the City of Hope, NIH/NCI grant (R01 CA229510-01), and Leukemia Lymphoma Society Clinical Scholar Award (2325–19) to C. Querfeld.
Author information
Authors and Affiliations
Contributions
Concept and design: X.U.M., J.P., M.L., S.T.R., C.Q.
Data acquisition: X.U.M., E.B., F.A.R., J.Z., S.T.R., C.Q.
Analysis and interpretation of the data: X.U.M., A.C., T.S., J.P., M.L., S.T.R., C.Q.
Drafting of the article or critical revision of the article for important intellectual content: all authors.
Study supervisor: C.Q.
Corresponding author
Ethics declarations
Ethics approval
The study was exempt from IRB review under 45CFR46.104 (d), and consent was not required. The study was approved for a Waiver of HIPAA Authorization (45CFR164.512(i)(2)(ii)).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
X.U.M., A.C., T.S., E.B.: none. J.P.: statistical consultant to Gilead Sciences; statistics instructor for ASTCT. M.L.: consultant to AstraZeneca. F.R.A.: consultant to Mallinckrodt; speaker’s bureau for Mallinckrodt; receives grant/research support from Johnson & Johnson, Elorac, Trillium, Stemline, MiRagen, Bioniz, and Mallinckrodt. J.Z.: consultant to Kyowa Kirin, Seattle Genetics, and Mundi Pharma. S.T.R.: speaker’s bureau for Celgene, Global Education Group and Paradigm Medical Communications, LLC, and Abbvie; consultant to Novartis Pharmaceuticals Corporation, Pepromene Bio, Inc, Exicure, and Apobiologix/Apotex Inc.; education advisory board to Seattle Genetics, NeoGenomics, and Aileron Therapeutics, Inc; has stock options with Pepromene Bio, Inc, and Exicure. C.Q.: Consultant to MiRagen, Helsinn/Actelion, Medvir, Stemline Therapeutics, Trillium, Bioniz, and Kyowa Kirin; contracted clinical investigator/researcher to MiRagen, Helsinn/Actelion, Bioniz, Kyowa Kirin, Celgene, Trillium, Esai, Soligenix, and Elorac; received research grant from Celgene.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(PDF 460 kb)
Rights and permissions
About this article
Cite this article
Martinez, X.U., Chowdhury, A., Stiller, T. et al. The impact of gender, age, race/ethnicity, and stage on quality of life in a spectrum of cutaneous lymphomas. Support Care Cancer 29, 6669–6679 (2021). https://doi.org/10.1007/s00520-021-06241-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06241-6