Abstract
Background
Literature data on the overuse and misuse of diagnostic procedures leading to end-of-life aggressiveness are scarce due to the limited amount of estimated economic waste. This study investigated the potential overuse of diagnostic procedures in a population of end-of-life patients.
Methods
This is a retrospective study on consecutive advanced patients admitted into two Italian hospices. Frequency and relative costs of X-ray imaging, CT scans, MRI, and interventional procedures prescribed in the 3 months before admission were collected in patient electronic charts and/or in administrative databases. We conducted a deeper analysis of 83 cancer patients with a diagnosis of at least 1 year before admission to compare the number of examinations performed at two distant time periods.
Results
Out of 541 patients, 463 (85.6%) had at least one radiological exam in the 3 months before last admission. The mean radiological exam number was 3.9 ± 3.2 with a relative mean cost of 278.60 ± 270.20 € per patient with a statistically significant (p < 0.001) rise near death. In the 86-patient group, a higher number of procedures was performed in the last 3 months of life than in the first quarter of the year preceding last admission (38.43 ± 28.62 vs. 27.95 ± 23.21, p < 0.001) with a consequent increase in cost.
Conclusions
Patients nearing death are subjected to a high level of “diagnostic aggressiveness.” Further studies on the integration of palliative care into the healthcare pathway could impact the appropriateness of interventions, quality of care, and, ultimately, estimated costs.
Similar content being viewed by others
Introduction
Literature studies have identified some factors as indicators of aggressive care and poor quality of cancer care near the end of life (EoL) [1]. Earle and colleagues categorized some indicators into three major areas: (a) access to the emergency room and admission to hospital or intensive care unit; (b) lack of or very late referral to hospice; and (c) overuse of chemotherapy very near death [2]. Other authors created scoring tools for quantifying the aggressiveness of care based on the prevalence of these indexes [3,4,5,6].
The increasing debate on how cancer patients are treated at EoL confirms that this topic is multidimensional and delicate. Palliative care has been identified as an appropriate means of addressing patient needs in this setting, positively impacting three major aspects: patient quality of life, healthcare service misutilization, and costs.
The rise of costs for cancer care worldwide [7] affects all the parties involved: patients, caregivers [8], and the healthcare system. In particular, the expenditure for cancer care in the USA was estimated in 2006 to rise from $104 to over $173 billion by 2020 [9, 10]. In Italy, as oncology care running costs are at risk of undermining healthcare sustainability, it is crucial to limit inappropriate interventions throughout the entire care pathway [11].
Medical oncologists can directly and indirectly determine most of the costs related to cancer care. For example, such expenditures can be reduced if diagnostic imaging is performed when a true benefit is shown, or if EoL discussions are promoted, as a result of good healthcare planning [9, 12, 13]. Literature data on the overuse and misuse of both imaging and invasive diagnostic procedures at EoL are currently scarce [14,15,16,17], probably due the limited amount of estimated economic waste. Previous studies have shown the considerable use of high-cost diagnostic imaging at EoL, with about one third of IV-stage-disease patients undergoing at least one high-cost imaging procedure in the last 30 days of life [15].
This study investigated the potential overuse of diagnostic procedures in a population of patients approaching EoL, with the aim of outlining the employed resources in such a challenging context [15], and defining what and how many procedures were performed nearer death than in earlier phases. In detail, we firstly counted the number of diagnostic procedures undergone by the population of two hospices in a retrospective study, estimating the associated costs. Secondly, in a subgroup of the same population, we compared the frequency of various procedures performed in 12 to 9 months before the last admission to hospice and in the 3 months before death, determining the respective costs.
Patients and methods
This retrospective study was conducted on a population of consecutive advanced cancer patients deceased in the hospices of the cities of Piacenza and Forlì (Emilia-Romagna Region) in the 3 months before hospice admission. The areas of Piacenza and Forlì are similar in terms of population and available health services. The study covered the period from January 2012 to June 2013 for Piacenza and from June 2013 to June 2014 for Forlì.
We collected the following information for each patient: age, sex, primary cancer site, length of hospice stay, and number of accesses to the Radiology Department. The frequency and type of diagnostic and interventional radiological exams performed on the patients in the 90 days before their admission to hospice were recorded and coded as: X-ray, computed tomography (CT) scan, magnetic resonance imaging (MRI), and interventional procedures (fine-needle biopsy of the lung, positioning of arterial catheter, ascending pyelography, biliary drainage, positioning of urethral stent). For analysis purposes, the hospice preadmission period was divided as follows: 90–61 days (M-3), 60–31 days (M-2), and 30–1 days (M-1) before admission. Frequency, type, and costs of examinations were calculated for each of the three periods. For a subgroup of patients from Forlì diagnosed with cancer up to 1 year before admission, an in-depth analysis was carried out for two distinct periods: 365–275 days (M12/9) and 90–1 days (M3/0) before admission.
In addition to X-ray, CT scan, MRI, and invasive procedures, we considered for analysis all healthcare procedures, such as ultrasound, electrocardiogram (ECG) and nuclear medicine tests, visits, hematological exams, radiotherapy, intravenous chemotherapy, and other therapeutic services. We reported the cost of each procedure performed in an outpatient setting. For the procedures performed in an inpatient setting, we estimated the costs of the overall inpatient stay. The Regional Healthcare Range of Fees table was used to determine the costs of the exams in both centers. The study was approved by the local ethics committee of each participating center.
Data sources
In both centers, patients were identified through hospice electronic patient charts, from which date of death was retrieved. In Piacenza, patients’ data were cross-checked with the Radiology Information System archive of the Radiology Department, which collects data from the three Radiology Units of the district public hospitals. In Forlì, patients’ data were linked with the regional administrative database of the Hospital Discharged Card (HDC) for inpatients, Specialistic Assistance (SA) for outpatients, and Emergency Room (ER) in order to retrieve all the performed radiological procedures. For the in-depth analysis at the hospice in Forlì, patients were selected by the date of diagnosis retrieved from the electronic health records and confirmed by the Cancer Registry, and subsequently matched to the administrative data through an automated and validated system of record linkage. All performed healthcare procedures were extracted from the HDC, SA and ER databases.
Statistical analysis
Patient characteristics, number of exam, and costs were expressed as mean ± standard deviations for continuous variables, and counts (%) for categorical variables. For continuous variables, also minimum and maximum values of distributions were reported. To evaluate the presence of an exponential growth over time for the number of the examined patients, the number and the cost of the exams performed in the 3 months before death, generalized linear models were estimated on natural logarithm of the three measures considering time as an independent variable. Wald p value tests for exponential trend were reported. A p value < 0.05 was considered statistically significant. For the analysis of the subgroup of patients with a cancer diagnosis of up to 1 year prior to hospice admission, absolute and relative variations were calculated. All statistical analyses were performed using Stata 14.1 for Windows (StataCorp LP, College Station, TX).
Results
We found a total of 541 deceased cancer patients in the two hospices during the considered periods with 17 ± 18 days of mean last hospice stay (range, 0–147). The general characteristics of the study population are reported in Table 1. Table 1 also shows that a total of 463 (85.6%) patients underwent ≥ 1 radiological exam in the 3 months before last admission. Of these, 76.5 and 9.1% underwent ≥ 2 and ≥ 6 radiological exams, respectively. The mean of radiological exams was 3.9 ± 3.2 per patient, with a total of 2091 radiological exams performed on 541 patients. If calculated on the 463 patients that underwent ≥ 1 exam, the mean number of exams per patient rose to 4.51 ± 3.0.
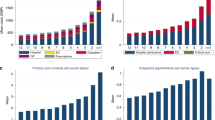
Even though the majority of performed exams was represented by X-ray (1198, 57.3%), 893 (42.7%) CT scans, MRI, and interventional exams were performed in the last 90 days before last admission to hospice. The total cost of the exams was 150,698.00 €. The distribution of the costs among the patients shows that for 162 (30%) cases, the cost was lower than 2780.65 €, whereas for 54 (10%) cases, the cost was higher than 45,758.60 €. The mean cost was 325.50 € per patient undergoing exams, and 278.60 per patient in the population (Fig. 1). The total cost for CT scans, MRI, and interventional exams was 124,924.60 €, accounting for 83.0% of the global expense. The mean number of exams and cost per patient in the two towns was similar (data not shown).
Subdividing the 90-day period in three periods of 1 month each (M-3, M-2, M-1) (Table 2) and examining the trend of the performed examinations, we noticed a clear increase in the trend approaching hospice admission. In fact, 37.3, 41.6, and 64.0% of patients performed ≥ 1 radiological exam at M-3, M-2, and M-1, respectively (p = 0.002). Also, the number of the exams performed increased nearing hospice admission: 446 (21.3%) at M-1, 625 (29.9%) at M-2, and 1020 (48.8%) at M-3 (p < 0.001). This trend was reflected in the mean number of exams performed per patient, which increased from less than 1 (0.8 ± 1.4) at M-3 to almost 2 (1.9 ± 2.3) at M-1. As shown in Table 2, the examined patients and number of radiological exams increased from M-3 to M-2 and from M-2 to M-1.
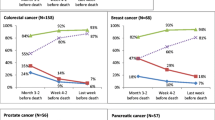
Table 3 shows that the total costs of the exams were 33,698.20 € at M-3, 51,088.80 € at M-2, and 65,911.00 € at M-1 and were significantly different (p < 0.001). The mean cost per patient was twofold higher at M-1 than at M-3 (62.30 € ± 143.00 € at M-3, 94.40 € ± 169.60 at M-2, and 121.80 € ± 181.80 € at M-1). The analysis of the use of the resources for the 86-patient group (55% females, mean age 67.23 ± 13.71 years) diagnosed with cancer up to 1 year before last hospice admission shows that the primary cancer sites were the gastrointestinal tract (22.1%), the breast (15.1%), the lung (14.0%), and others (48.8%). At M3/0, the total number of performed procedures was 3305, of which 2613 (79.1%) were performed on outpatients, and 692 (20.9%) on inpatients. At M12/9, the total number of performed procedures was 2404, of which 2008 (83.5%) were performed on outpatients and 396 (16.5%) on inpatients. We observed a higher number of procedures performed in the inpatient than in the outpatient setting from M12/9 to M3/0 with an absolute increase of 901 exams (relative increase +37.5%) nearing death. The mean number of exams (±SD) ranged from 27.95 ± 23.21 at M12/9 to 38.43 ± 28.62 at M3/0. As regards the costs, we found that before the last admission they amounted to 735,990.70 € at M3/0 and to 553,814.10 € at M12/9, with an absolute difference of 182,176.50 €, and a relative increase of the global expenses by 32.9% approaching admission (Table 4).
Discussion
Our results confirmed the increase in the number of diagnostic exams carried out in the last months of a patient’s life, which, however, did not always offer an advantage in terms of survival [6, 18]. In our study, considering the number of patients undergoing at least one radiological exam in the 90 days before last admission to hospice, we found that both the number of exams and the relevant costs had a statistically significant increase approaching death. A total of 37.3 and 64.0% of patients underwent at least one radiological exam up to 3 months and up to 1 month before hospice admission, respectively.
As imaging procedures have no diagnostic purpose in advanced patients, they can be justified only in view of the management of acute symptoms, evaluation of disease progression, and assessment of treatment effect. The latter can be useful for evaluating whether to discontinue and/or change the treatment line [6, 14, 15, 18]. The use of diagnostic imaging, however, is still widespread despite the present guidelines [19]. In particular, we observed that it did not reduce as death was nearing; rather, it increased, resulting in higher healthcare costs. We also noticed a rising number of diagnostic invasive procedures from M3 to M1, even if the absolute number was low. The role of these procedures is as yet unclear. On the one hand, given that their palliative purpose supports their appropriateness at EoL [20], we would expect a more intensive use than that seen in our case mix. On the other hand, their invasiveness and potential comorbidity should be carefully evaluated against any real therapeutic benefit [15].
We firmly believe that costs for healthcare services in the last year of life are high, despite the fact that a recent study has advocated the “myth” of a costly EoL care, which would represent only a minority of the total annual expenditure in the USA [21]. However, most authors agree in considering the costs of EoL care a major item of healthcare expenditure. Comparing data from the last 3 months of life with those from 9 months back to 1 year before death in our subgroup analysis, we found that a higher number of procedures were performed at M3/0 for almost all the considered procedures, with higher corresponding costs. Chemotherapy and radiotherapy administrations were the only procedures that were reasonably reduced by M3/0, as the former is usually avoided at EoL and the latter is considered appropriate only in a few cases. Conversely, the percentage of invasive procedures, such as X-ray, ultrasound, ECG, and nuclear medicine tests, showed an increase at EoL. From a methodological point of view, we adapted Yabroff’s [22] timeframe definitions (M12/9 and M3/0), interpreting M12/9 as the “continuous care phase” and M3/0 as the “EoL phase.” We acknowledge, however, that it is quite difficult to make any direct comparison with other studies on costs at EoL due to the different timeframes adopted (i.e., 3 days [23], 7 days [24], or 30 days [14] before death).
The main limitation of this retrospective study is the lack of any information about the clinical indications or the patient’s preferences for these procedures. These administrative databases, however, do not collect data from palliative care settings but from inpatient and outpatient settings. As all the considered procedures at EoL were not prescribed within a palliative care context, we observed a high use of resources and questionable clinical appropriateness. Hospital-based consultation teams and interdisciplinary palliative care teams in vertically integrated healthcare organizations can both improve the appropriateness of procedures and reduce costs [25]. Ideally, early palliative care (inpatient consultation teams and outpatient clinics) should be closely integrated with EoL palliative care (home palliative care and hospice services), since it can indirectly lower healthcare costs by favoring timely referrals to hospice, which results in reduced hospitalization, readmission, intensive care unit and emergency room access, and, possibly, intravenous chemotherapy in the last month of life.
In our retrospective study, we observed that an increasing number of diagnostic procedures were performed at EoL. Being administered in the very last part of life, this kind of diagnostic aggressiveness yielded no evaluable therapeutic results.
A subgroup of patients resident in the same area with a diagnosis of up to 1 year before admission was subjected to a higher number of interventions at M3/0 than at M12/9. These data could elicit further studies on the extent of the role of a palliative care network in determining the appropriateness of interventions, quality of life, quality of care, and, ultimately, costs, so that healthcare resources can be better employed for interventions of higher-benefit levels for patients.
References
Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block SJ (2003) Identifying potential indicators of the quality of end-of-life cancer care from administrative data. Clin Oncologia 21(6):1133–1138
Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ (2008) Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol 26(23):3860–3866. https://doi.org/10.1200/JCO.2007.15.8253
Tang ST, Wu SC, Hung YN, Chen JS, Huang EW, Liu TW (2009) Determinants of aggressive end-of-life care for Taiwanese cancer decedents, 2001 to 2006. J Clin Oncol 27(27):4613–4618. https://doi.org/10.1200/JCO.2008.20.5096
Hui D, Didwaniya N, Vidal M, Shin SH, Chisholm G, Roquemore J, Bruera E (2014) Quality of end-of-life care in patients with hematologic malignancies: a retrospective cohort study. Cancer 120(10):1572–1578. https://doi.org/10.1002/cncr.28614
Hui D, Kim SH, Roquemore J, Dev R, Chisholm G, Bruera E (2014) Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer 120(11):1743–1749. https://doi.org/10.1002/cncr.28628
Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC (2004) Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22(2):315–321. https://doi.org/10.1200/JCO.2004.08.136
Dalal S, Bruera E (2017) End-of-life care matters: palliative cancer care results in better care and lower costs. Oncologist 22(4):361–368. https://doi.org/10.1634/theoncologist.2016-0277
Cohen RA, Kirzinger WK (2014) Financial burden of medical care: a family perspective. NCHS Data Brief 142:1–8
Smith TJ, Hillner BE (2011) Bending the cost curve in cancer care. N Engl J Med 364(21):2060–2065. https://doi.org/10.1056/NEJMsb1013826
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML (2011) Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 103(2):117–128. https://doi.org/10.1093/jnci/djq495
Massa I, Balzi W, Burattini C, Gentili N, Bucchi L, Nanni O, Gallegati D, Pierini A, Amadori D, Falcini F, Altini M (2017) The challenge of sustainability in healthcare systems: frequency and cost of inappropriate patterns of breast cancer care (the E.Pic.A study). Breast 34:103–107. https://doi.org/10.1016/j.breast.2017.05.007
Dow LA, Matsuyama RK, Ramakrishnan V, Kuhn L, Lamont EB, Lyckholm L, Smith TJ (2010) Paradoxes in advance care planning: the complex relationship of oncology patients, their physicians, and advance medical directives. J Clin Oncol 28(2):299–304. https://doi.org/10.1200/JCO.2009.24.6397
Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, Mitchell SL, Jackson VA, Block SD, Maciejewski PK, Prigerson HG (2008) Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300(14):1665–1673. https://doi.org/10.1001/jama.300.14.1665
Hu YY, Kwok AC, Jiang W, Taback N, Loggers ET, Ting GV, Lipsitz SR, Weeks JC, Greenberg CC (2012) High-cost imaging in elderly patients with stage IV cancer. J Natl Cancer Inst 104(15):1164–1172. https://doi.org/10.1093/jnci/djs286
Kwok AC, Hu YY, Dodgion CM, Jiang W, Ting GV, Taback N, Lipsitz SR, Weeks JC, Greenberg CC (2015) Invasive procedures in the elderly after stage IV cancer diagnosis. J Surg Res 193(2):754–763. https://doi.org/10.1016/j.jss.2014.08.021
Dinan MA, Curtis LH, Hammill BG, Patz EF Jr, Abernethy AP, Shea AM et al (2010) Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999-2006. JAMA 303(16):1625–1631. https://doi.org/10.1001/jama.2010.460
Belloni E, Tentoni S, Cella A, Cassinelli D, Bertè R, Scagnelli P (2017) Radiological exams on end-stage oncologic patients before hospice admission. Radiol Med 222(10):792–797
Matsuyama R, Reddy S, Smith TJ (2006) Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol 24(21):3490–3496
Sirovich B, Gallagher PM, Wennberg DE, Fisher ES (2008) Discretionary decision making by primary care physicians and the cost of U.S. health care. Health Aff (Millwood) 27(3):813–823. https://doi.org/10.1377/hlthaff.27.3.813
Sato KT, Takehana C (2007) Palliative nonvascular interventions. Semin Intervent Radiol 24(4):391–397. https://doi.org/10.1055/s-2007-992327
Aldridge MD, Kelley AS (2015) The myth regarding the high cost of end-of-life care. Am J Public Health 105(12):2411–2415. https://doi.org/10.2105/AJPH.2015.302889
Yabroff KR, Lamont EB, Mariotto A (2008) Cost of care for elderly cancer patients in the United States. JNCI 100(9):630–641. https://doi.org/10.1093/jnci/djn103
West E, Costantini M, Pasman HR, Onwuteaka-Philipsen B (2014) A comparison of drugs and procedures of care in the Italian hospice and hospital settings: the final three days of life for cancer patients. BMC Health Serv Res 14(1):496. https://doi.org/10.1186/s12913-014-0496-2
Dasch B, Kalies H, Feddersen B, Ruderer C, Hiddemann W, Bausewein C (2017) Care of cancer patients at the end of life in a German university hospital: a retrospective observational study from 2014. PLoS One 12(4):e0175124. https://doi.org/10.1371/journal.pone.0175124
Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, Firn JI, Paice JA, Peppercorn JM, Phillips T, Stovall EL, Zimmermann C, Smith TJ (2017) Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35(1):96–112. https://doi.org/10.1200/JCO.2016.70.1474
Acknowledgements
The authors thank Veronica Zanoni for editorial assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Massa, I., Balzi, W., Altini, M. et al. The challenge of sustainability in healthcare systems: frequency and cost of diagnostic procedures in end-of-life cancer patients. Support Care Cancer 26, 2201–2208 (2018). https://doi.org/10.1007/s00520-018-4067-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4067-7