Abstract
Purpose
Participation in cancer cachexia clinical trials requires a defined weight loss (WL) over time. A loss in skeletal muscle mass, measured by cross-sectional computed tomography (CT) image analysis, represents a possible alternative. Our aim was to compare WL versus muscle loss in patients who were screened to participate in a cancer cachexia clinical trial.
Methods
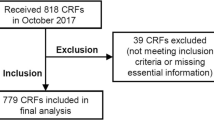
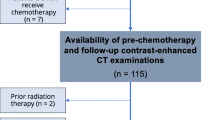
This was a single-center, retrospective analysis in metastatic colorectal cancer patients screened for an interventional cancer cachexia trial requiring a ≥5 % WL over the preceding 6 months. Concurrent CT images obtained as part of standard oncology care were analyzed for changes in total muscle and fat (visceral, subcutaneous, and total).
Results
Of patients screened (n = 36), 3 (8 %) enrolled in the trial, 17 (47 %) were excluded due to insufficient WL (<5 %), 3 (8 %) were excluded due to excessive WL (>20 %), and 16 (44 %) met inclusion criteria for WL. Patients who met screening criteria for WL (5–20 %) had a mean ± SD of 7.7 ± 8.7 % muscle loss, 24.4 ± 37.5 % visceral adipose loss, 21.6 ± 22.3 % subcutaneous adipose loss, and 22.1 ± 24.7 % total adipose loss. Patients excluded due to insufficient WL had 2 ± 6.4 % muscle loss, but a gain of 8.5 ± 39.8 % visceral adipose, and 4.2 ± 28.2 % subcutaneous adipose loss and 0.8 ± 28.4 % total adipose loss. Of the patients excluded due to WL <5 % (n = 17), 7 (41 %) had a skeletal muscle loss >5 %.
Conclusions
Defining cancer cachexia by WL over time may be limited as it does not capture skeletal muscle loss. Cross-sectional CT body composition analysis may improve early detection of muscle loss and patient participation in future cancer cachexia clinical trials.


Similar content being viewed by others
References
Barret M, Antoun S, Dalban C, et al. (2014) Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 66:583–589
Gray C, MacGillivray TJ, Eeley C, et al. (2011) Magnetic resonance imaging with k-means clustering objectively measures whole muscle volume compartments in sarcopenia/cancer cachexia. Clin Nutr 30:106–111
Prado CM, Baracos VE, McCargar LJ, et al. (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Stene GB, Helbostad JL, Amundsen T, et al. (2015) Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol 54:340–348
Utech AE, Tadros EM, Hayes TG, et al. (2012) Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachex Sarcopenia Muscle 3:245–251
von Haehling S, Anker SD (2010) Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachex Sarcopenia Muscle 1:1–5
Tisdale MJ (1997) Biology of cachexia. J Natl Cancer Inst 89:1763–1773
Temel JS, Abernethy AP, Currow DC, et al (2016) Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol
Strasser F, Luftner D, Possinger K, et al. (2006) Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-in-Cachexia-Study-Group. J Clin Oncol 24:3394–3400
Lieffers JR, Mourtzakis M, Hall KD, et al. (2009) A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr 89:1173–1179
Roubenoff R, Kehayias JJ, Dawson-Hughes B, et al (1993) Use of dual-energy X-ray absorptiometry in body-composition studies: not yet a ‘gold standard’. Am J Clin Nutr (USA)
Mourtzakis M, Prado CMM, Lieffers JR, et al. (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Prado CM, Birdsell LA, Baracos VE (2009) The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 3:269–275
Shen W, Punyanitya M, Wang Z, et al. (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 97:2333–2338
Shen W, Punyanitya M, Wang Z, et al. (2004) Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr 80:271–278
Heymsfield SB, Smith R, Aulet M, et al. (1990) Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 52:214–218
Fearon K, Strasser F, Anker SD, et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495
Fearon K, Argiles J, Baracos V, et al. (2015) Request for regulatory guidance for cancer cachexia intervention trials. J Cachex Sarcopenia Muscle 6:272–274
Crawford J, Prado CM, Johnston MA, et al. (2016) Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER trials). Curr Oncol Rep 18:1–11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Roeland, E.J., Ma, J.D., Nelson, S.H. et al. Weight loss versus muscle loss: re-evaluating inclusion criteria for future cancer cachexia interventional trials. Support Care Cancer 25, 365–369 (2017). https://doi.org/10.1007/s00520-016-3402-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3402-0