Abstract
Purpose
Low Karnofsky performance status (KPS) and elevated lactate dehydrogenases (LDHs) as a surrogate marker for tumor load and cell turnover may depict patients with a very short life expectancy. To validate this finding and compare it to other indices, namely, the recursive partitioning analysis (RPA) and diagnosis-specific graded prognostic assessment (DS-GPA), a multicenter analysis was undertaken.
Methods
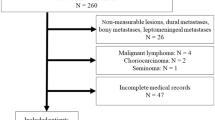
A retrospective analysis of 234 metastatic melanoma patients uniformly treated with palliative whole brain radiotherapy (WBRT) was done. Univariate and multivariate analyses were used to determine the impact of patient-, tumor-, and treatment-related parameters on overall survival (OS).
Results
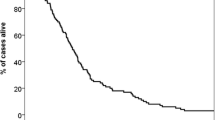
KPS and LDH emerged as independent factors predicting OS. By combining KPS and LDH values (KPS/LDH index), groups of patients with statistically significant differences in median OS (days; 95 % CI) after onset of WBRT were identified: group 1 (KPS ≥70/normal LDH) 234 (96–372), group 2 (KPS ≥70/elevated LDH) 112 (69–155), group 3 (KPS <70/normal LDH) 43 (12–74), and group 4 (KPS <70/elevated LDH) 29 (17–41). Between all four groups, statistically significant differences were observed. The RPA and DS-GPA indices failed to distinguish significantly between good and moderate prognosis and were inferior in predicting a very unfavorable prognosis.
Conclusions
The parameters KPS and LDH independently impacted on OS. The combination of both (KPS/LDH index) identified patients with a very short life expectancy, who might be better served by recommending best supportive care instead of WBRT. The KPS/LDH index is simple and effective in terms of time and cost as compared to other prognostic indices.



Similar content being viewed by others
References
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973–2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872
Daryanani D, Plukker JT, de Jong MA et al (2005) Increased incidence of brain metastases in cutaneous head and neck melanoma. Melanoma Res 15(2):119–124
Donizy P, Kaczorowski M, Leskiewicz M et al (2014) Mitotic rate is a more reliable unfavourable prognosticator than ulceration for early cutaneous melanoma: a 5-year survival analysis. Oncol Rep 32(6):2735–2743
Amer MH, Al-Sarraf M, Vaitkevicius VK (1979) Clinical presentation, natural history and prognostic factors in advanced malignant melanoma. Surg Gynecol Obstet 149:687–692
Atkinson L (1978) Melanoma of the central nervous system. Aust N Z J Surg 48:14–16
Mc Neer G, Das Gupta T (1965) Problem of recurrence in the management of melanoma. CA Cancer J Clin 15:270–274
Rades D, Gerdan L, Segedin B et al (2013) Brain metastasis. Prognostic value of the number of involved extracranial organs. Strahlenther Onkol 189:996–1000
Gaspar L, Scott C, Rotman M et al (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:45–751
Sperduto PW, Berkley B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510–514
Sperduto PW, Chao ST, Sneed PK et al (2010) Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4259 patients. Int J Radiat Oncol Biol Phys 77:655–661
Partl R, Richtig E, Avian A, Berghold A, Kapp KS (2013) Karnofsky performance status and lactate dehydrogenase predict the benefit of palliative whole-brain irradiation in patients with advanced intra- and extracranial metastases from malignant melanoma. Int J Radiat Oncol Biol Phys 85:662–666
Staudt M, Lasithiotakis K, Leiter U, Meier F, Eigentler T, Bamberg M, Tatagiba M, Brosart P, Garbe C (2010) Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer 102:1213–1218
Dziggel L, Segedin B, Podvrsnik NH, Oblak I, Schild SE, Rades D (2014) A survival score for patients with brain metastases from less radiosensitive tumors treated with whole-brain radiotherapy alone. Strahlenther Onkol 190:54–58
Chatani M, Teshima T, Hata K, Inoue T (1986) Prognostic factors in patients with brain metastases from lung carcinoma. Strahlenther Onkol 162:157–161
Chatani M, Matayoshi Y, Masaki N, Inoue T (1994) Radiation therapy for brain metastases from lung carcinoma. Prospective randomized trial according to the level of lactate dehydrogenase. Strahlenther Onkol 170:155–161
Eigentler TK, Figl A, Krex D et al (2011) Number of metastases, serum lactate dehydrogenase level, and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer 117:1697–1703
Nieder C, Marienhagen K, Dalhaug A, Norum J (2012) Towards improved prognostic scores predicting survival in patients with brain metastases: a pilot study of serum lactate dehydrogenase levels. Sci World J 2012:609323. doi:10.1100/2012/609323
Tsao MN, Lloyd N, Wong RK, Chow E, Rakovitch E, Laperriere N, Xu W, Sahgal A (2012) Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev 4:CD003869. doi:10.1002/14651858.CD003869.pub3, Review
Gondi V, Pugh SL, Tome WA (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32(34):3810–3816. doi:10.1200/JCO.2014.57.2909
Long GV, Margolin KA (2013) Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am Soc Clin Oncol Educ Book 393–8. doi: 10.1200/EdBook_AM.2013.33.393.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Partl, R., Fastner, G., Kaiser, J. et al. KPS/LDH index: a simple tool for identifying patients with metastatic melanoma who are unlikely to benefit from palliative whole brain radiotherapy. Support Care Cancer 24, 523–528 (2016). https://doi.org/10.1007/s00520-015-2793-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2793-7