Abstract
Purpose
Paclitaxel-based chemotherapy continues to be an integral component of breast cancer treatment. Prolonged use of paclitaxel may result in repeated doses of premedications that can have unwanted side effects. Infusion hypersensitivity reactions occurring beyond the second dose of paclitaxel are infrequent and not well characterized. We previously published the results of a small, prospective pilot trial demonstrating the safety and feasibility of discontinuing premedications in patients who received the first two doses of paclitaxel-based chemotherapy without experiencing an infusion hypersensitivity reaction. In this study, we aimed to retrospectively characterize the incidence of rescue medication using this abbreviated premedication regimen in our institution following the publication of the pilot study.
Methods
Patients with stages I–IV breast cancer who received paclitaxel from January 2011 through June 2013 were screened for eligibility. Patients who did not experience an infusion hypersensitivity reaction with their first or second dose of paclitaxel and discontinued paclitaxel premedication for subsequent doses were included in this analysis. The primary endpoint was to estimate the incidence of rescue medication use for the treatment of paclitaxel infusion hypersensitivity during doses three to six of paclitaxel in the study population.
Results
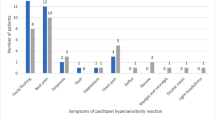
In total, 449 patients received paclitaxel-based chemotherapy for the treatment of breast cancer during the interval time period. After receiving the first two doses of paclitaxel-based chemotherapy without experiencing an infusion hypersensitivity reaction, 234 breast cancer patients had their premedications discontinued for all remaining paclitaxel doses. These patients tolerated future paclitaxel doses without severe or life-threatening complications related to infusion hypersensitivity. The majority of patients did not have any symptoms of an infusion reaction, with only two of these patients requiring rescue medication to treat an infusion hypersensitivity reaction with subsequent paclitaxel doses (0.85; 95 % confidence interval (CI), 0.10–3.05 %).
Conclusions
Discontinuation of paclitaxel premedications in breast cancer patients who have not experienced an infusion hypersensitivity reaction with the first two doses of paclitaxel is not associated with increased rate of rescue medication use for infusion hypersensitivity.
Similar content being viewed by others
References
Taxol® injection (package insert). Princeton, NJ: Bristol-Myers Squibb Company; July 2007
Sparano JA et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358(16):1663–1671
Miller K et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676
Markman M et al (1999) An effective and more convenient drug regimen for prophylaxis against paclitaxel-associated hypersensitivity reactions. J Cancer Res Clin Oncol 125(7):427–429
Bookman MA et al (1997) Intravenous prophylaxis for paclitaxel-related hypersensitivity reactions. Semin Oncol 24(6 Suppl 19):S19-13–S19-15
Kloover JS et al (2004) Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of pre-medication regimens. Br J of Cancer 90:304–305
Micromedex. Diphenhydramine. v 1.0 2007. Available at: http://www.thomsonhc.com/micromedex2/librarian. Accessed March 2007
Quock J et al (2002) Premedication strategy for weekly paclitaxel. Cancer Invest 20(5–6):666–672
Koppler H et al (2001) Dose reduction of steroid premedication for paclitaxel: no increase of hypersensitivity reactions. Onkologie 24(3):283–285, English, German
Braverman AS et al (2005) Tapering and discontinuation of glucocorticoid prophylaxis during prolonged weekly to biweekly paclitaxel administration. Chemotherapy 51(2–3):116–119
Lenz HJ (2007) Management and preparedness for infusion and hypersensitivity reactions. Oncologist 12(5):601–609
Berger MJ et al (2012) Feasibility of stopping paclitaxel premedication after two doses in patients not experiencing a previous infusion hypersensitivity reaction. Support Care Cancer 20(9):1991–1997
Markman M et al (2000) Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol 18(1):102–105
Zanotti KM, Markman M (2001) Prevention and management of antineoplastic-induced hypersensitivity reactions. Drug Saf 24(10):767–779
Feldweg AM et al (2005) Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol 96(3):824–829
Disclaimers
None.
Research funding
None.
Previous publication of manuscript
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berger, M.J., Vargo, C., Vincent, M. et al. Stopping paclitaxel premedication after two doses in patients not experiencing a previous infusion hypersensitivity reaction. Support Care Cancer 23, 2019–2024 (2015). https://doi.org/10.1007/s00520-014-2556-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2556-x