Summary
Aim
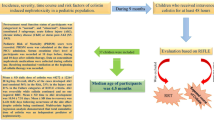
The aim of this study was to identify the predictors of acute renal injury associated with colistin treatment.
Methods
The patients who received treatment with colistin for more than 3 days were included in this retrospective cohort study. Acute renal injury was defined by the RIFLE (Risk Injury Failure Loss End stage renal disease) criteria. Patients whose serum creatinine levels increased at least 1.5-fold compared with baseline value were considered as cases with renal injury. The independent variables determining the development of acute renal injury were investigated by survival analysis.
Results
A total of 112 cases [67 (59.8 %) were male, median age 64 (range: 18–93) years] were included in the study. Acute renal injury occurred in 66 (58.9 %) patients. Renal injury developed in first 7 days of the colistin therapy in 52 (78.8 %) cases and at day 8–23 in 14 (21.2 %) cases. On the day with highest levels of creatinine, 25 (22.3 %), 17 (15.2 %), and 33 (29.5 %) cases were in ‘Risk’, ‘Injury’, and ‘Failure’ group, respectively, according to RIFLE criteria. We identified three independent risk factors predicting acute colistin-induced renal injury: advanced age, low serum albumin levels, and high serum total bilirubin levels [odds ratio (confidence interval) = 1.022 (1.006–1.037), 0.643 (0.415–0.994), and 1.129 (1.014–1.257), respectively].
Conclusions
The advanced age, low serum albumin levels, and high serum total bilirubin levels are independent risk factors for colistin-induced nephrotoxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Multidrug-gram-negative bacilli infections continue to be a serious problem worldwide [1]. Colistin is usually available as almost only treatment option for these infections. Most important problem during colistin therapy is development of renal injury [2–22]. Knowledge of the factors involved in occurrence of colistin-associated renal injury may be important to take measures preventing the development of renal injury [2, 4, 6–8, 11, 22–25]. The aim of this study is to investigate the rates of development of colistin-associated renal impairment and variables determining the development of renal injury.
Patients, materials, and methods
This is a retrospective cohort study. Study was conducted by reviewing the records of adult in-patients who received parenteral colistin therapy between January 2011 and December 2012 at two different centers in the same city.
Patient selection
Patients with no renal injury at the baseline and received colistin for at least 3 days during their treatment (colistimethate sodium) and had daily creatinine monitoring were included in study. Patients below 18 years of age, with a history of renal impairment, with serum creatinine levels exceeding upper limit of normal (> 1.3 mg/dl) prior to colistin therapy, had received colistin therapy less than 3 days, and who lacked daily creatinine measurements after initiation of colistin therapy were excluded from the study. The first colistin therapy was evaluated for patients who had received multiple colistin therapies.
Definition of development of renal injury
Nephrotoxicity was defined according to RIFLE (Risk Injury Failure Loss End stage renal disease) criteria (Table 1) [6]. Patients whose serum creatinine levels increased at least 1.5-fold compared with baseline value were considered as cases with renal injury. The number of cases in ‘Risk’, ‘Injury’, and ‘Failure’ group according to RIFLE criteria were determined at the day on which the levels of creatinine were highest.
Variables evaluated in study
Information on age, gender, presence of diabetes, drugs received during colistin therapy and duration of use, use of intravenous contrast agent and number of use, use of inhaled colistin, score of Glasgow Coma Scale [27], prothrombin time, serum total bilirubin, and albumin levels before colistin therapy were obtained from the patient files. Among drugs co-administered with colistin therapy, information was obtained particularly for drugs belonging to glycopeptides group, calcineurin inhibitors, nonsteroidal anti-inflammatory drugs, trimethoprim-sulphometoxasole, aminoglycoside, rifampisin, amphotericine B, and intravenous contrast agents and use of these drugs were considered if received during or up to 1 week before colistin therapy at the latest.
Study protocol
Cases were divided into two groups as patients with and without renal injury, and independent variables determining the development of renal injury were investigated by survival analysis.
Statistical analysis
SPSS-17 (SPSS Inc., Chicago, IL) statistical package program was used to evaluate the data obtained from study. Categorical variables were expressed as number of cases and percentage, whereas the continuous variables with abnormal distribution were expressed as median (minimum–maximum) value. Univariate Cox regression analysis was used to investigate the variables determining the development of renal impairment. Variables determined as significant (p < 0.05) in univariate analysis were included in multivariate Cox regression analysis (enter method) and independent variables determining the development of colistin-associated renal injury were investigated. All statistical tests were performed two-sided and value of p < 0.05 was considered as significant.
Ethic committee approval was not obtained because this was a retrospective study.
Results
A total of 112 cases were included in this study [67 (59.8 %) were men, median age was 64 years (range: 18–93)]. In all, 99 patients (88.4 %) received colistin treatment in intensive care unit and 13 (11.6) received in ward. Of the 112 cases, 83 (74.1 %), 12 (10.7 %), 8 (7.1 %), 5 (4.5 %), and 4 (3.6 %) were diagnosed as ventilator-associated pneumonia, bacteremia, soft tissue infection, central venous catheter-related infection, and urinary tract infection, respectively and colistin therapy was initiated. Acinetobacter baumannii, Pseudomonas aeruginosa, combined Acinetobacter baumannii and Pseudomonas aeruginosa, and Enterobacteriaceae were isolated as causative microorganism in 68 (60.7 %), 20 (17.9 %), 16 (14.3 %), and 8 (7.1 %) of the cases, respectively. Colistin was administered at a daily dose of 5 mg/ideal body weight. A loading dose has not been given to any patients. Development of renal injury was observed in 66 (58.9 %) cases. On the day with highest levels of creatinine, 25 (22.3 %), 17 (15.2 %), and 33 (29.5 %) cases were in ‘Risk’, ‘Injury’, and ‘Failure’ group, respectively, according to RIFLE criteria. Renal impairment developed during first 7 days of the colistin therapy in 52 (78.8 %) cases and at day 8–23 in 14 (21.2 %) cases. Data on characteristics of cases with and without renal injury is summarized in Table 2. There were no patient using nephrotoxic drugs co-administered with colistin therapy. Multivariate Cox regression analysis showed that advanced age, low serum albumin levels, and high serum total bilirubin levels were independent variables determining colistin-associated renal injury (Table 3).
Discussion
In literature, the rates of development of colistin-associated renal injury were found to be varied between 0 and 60 % based on the different definition criteria of renal injury and different patient population [2–22]. We believe that this is due to difference in definition criteria of renal impairment in the studies and difference in risk factors of nephrotoxicity among the patient groups. Colistin-associated renal injury was found at the rates of 43–66 % in studies where definition of renal impairment was made according to RIFLE criteria [2–4]. Rates of renal injury are expected to be lower in studies defining the renal injury based on a higher creatinine level. For example, colistin-associated renal injury was observed at lower rates of 8.3–34 % in studies defining the renal injury as serum creatinine level higher than 2 mg/dl [7, 8, 11, 12, 14]. In our study, the rate of renal injury was similar with the studies, which defined the renal injury according to RIFLE criteria. In the literature, 11–15 %, 5–17 %, 13–80 % of the cases was demonstrated to be in ‘Risk’, ‘Injury’, and ‘Failure’ group, respectively, with colistin therapy according to RIFLE criteria [2, 3, 6]. Similar rates are also applicable for our cases. Colistin nephrotoxicity is reported to be observed at early stage in two studies performed [11, 23]. In one of these studies, nephrotoxicity was reported within first 7 days in 70.7 % of the cases with nephrotoxicity, and this rate is very close to the rate observed in our study [25]. This finding contradicts with the findings of a limited number of studies suggesting that the longer duration of use and higher dose of total colistin increase nephrotoxicity [23, 24]. Higher frequency of early development of nephrotoxicity in patients who had used colistin in our study appears to be more consistent with studies suggesting that the duration of colistin use and total colistin dose do not increase nephrotoxicity [3, 4, 22].
In literature, variables that may determine the development of colistin-associated renal injury were suggested as advanced age, long-term colistin use, excessive cumulative dose of colistin, low level of serum albumin, high level of serum bilirubin, presence of diabetes, and co-administration of other nephrotoxic drugs with colistin [2, 4, 6–8, 11, 22–25].
Our study showed that the colistin nephrotoxicity is more common in patients with advanced age. In literature, two studies suggested that the advanced age increases the risk of colistin-associated nephrotoxicity [6, 22], whereas many studies did not find such relationship [2–4, 7, 8, 11, 23, 24]. It is known that the mitochondrial functions are impaired with the advanced age and thus, reactive oxygen radicals are accumulated in body [28]. Accumulated reactive oxygen radicals disrupt the functions of many cells [29]. Key role of the increased oxidative stress in colistin-associated nephrotoxicity was demonstrated in experimental studies with rats [30, 31]. Prevention of colistin-associated nephrotoxicity by ascorbic acid, an antioxidant, was suggested to be an evidence of this finding [32]. We believe that higher frequency of colistin nephrotoxicity in advanced ages is due to the susceptibility of elders to the effect of colistin to produce increased reactive oxygen radicals in kidneys.
Two studies showed that the low level of serum albumin is a risk factor for colistin nephrotoxicity [3, 8]. One study observed no relationship between serum albumin levels and nephrotoxicity [2]. It is known that colistin binds to proteins by 55 % affinity in blood circulation [33]. The increase observed in free drug level with the decrease in blood albumin levels appears to increase the risk of nephrotoxicity. Low level of blood albumin is demonstrated to be a risk factor also for aminoglycoside toxicity such as the case for colistin. In addition, the severity of underlying disease of the patient may cause both decrease in serum albumin levels and development of nephrotoxicity. In our study, serum albumin levels were observed to be lower in patients with colistin nephrotoxicity than patients without colistin nephrotoxicity.
There is a study suggesting that the colistin nephrotoxicity is observed more commonly in patients with high serum bilirubin levels, whereas another study suggested that the risk of nephrotoxicity is not changed [3, 6]. Hyperbilirubinemia has been demonstrated to increase aminoglycoside-associated nephrotoxicity [34, 35]. Similarly, increased bilirubin and bile salts may increase nephrotoxicity in patients using colistin. In addition, probably, patients with severe organ failure may develop both renal dysfunction and hyperbilirubinemia. In our study, serum bilirubin levels were observed to be higher in patients with colistin nephrotoxicity than patients without colistin nephrotoxicity.
There are several studies suggesting that the colistin nephrotoxicity is increased in patients who had received high daily dose of colistin or high total dose of colistin and had used colistin for long term, as well as studies suggesting that the nephrotoxicity is not changed based on these factors [2–4, 11, 22–24]. As treatment was immediately stopped in many cases who had developed colistin-associated nephrotoxicity in our study, total dose of colistin was found to be lower in patients with nephrotoxicity than the patients without nephrotoxicity; thus, the effect of total dose of colistin on nephrotoxicity could not be determined.
A limited number of studies demonstrated that the risk of nephrotoxicity is increased in patients using nonsteroidal anti-inflammatory drugs and antibiotics belonging to aminoglycoside and glycopeptide group with colistin, while others demonstrated that the risk of nephrotoxicity is not changed [2–4, 7, 8, 11]. Use of intravenous contrast agent, development of hypotension, and use of vasopressor drugs have not been shown to increase colistin nephrotoxicity [3, 4, 8, 11]. Our study, as distinct from other studies, included the duration of co-administration of nephrotoxic drugs with colistin in variables affecting the nephrotoxicity rather than including nephrotoxic drug use only. No relationship has been found between co-administration of nephrotoxic drugs with colistin and nephrotoxicity in our study.
Cases were divided into two groups in a study, and in addition to antibiotics, inhaled colistin was added in treatment for one group, while inhaled normal saline was added in treatment for other group and no difference was observed for nephrotoxicity between the two groups [36]. Also in our study, it was observed that the addition of inhaled colistin to colistin therapy do not increase nephrotoxicity.
In our study, it did not increase the rate of colistin-induced nephrotoxicity to be in intensive care unit. We believe that this finding is due to the small number of patients in intensive care unit.
The most important weakness of our study include its retrospective design and absence of a control group. According to literature data, most of the studies comparing the case groups which received colistin with control groups which received antibiotics other than colistin have found no difference in terms of nephrotoxicity between two groups [5, 13, 14, 17, 18, 20, 37–39]. However, the most prominent feature of these studies is their limited power because of the limited number of cases. In two studies, which included much more cases than these studies, nephrotoxicity has been shown to be observed more commonly in cases using colistin compared with cases using antibiotics other than colistin [10, 21]. Although our study did not include a control group, renal injury developed in more than half of the cases after initiation of colistin therapy suggesting that colistin has an important effect in development of renal injury.
Findings of this study suggested that the advanced age, low serum albumin levels, and high serum total bilirubin levels are important risk factors for colistin-induced nephrotoxicity.
References
Waterer GW, Wunderink RG. Increasing threat of gram-negative bacteria. Crit Care Med. 2001;29(4):75–81.
Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis. 2011;53(9):879–84.
Kwon JA, Lee JE, Huh W, et al. Predictors of acute kidney injury associated with intravenous colistin treatment. Int J Antimicrob Agents. 2010;35(5):473–7.
Kubin CJ, Ellman TM, Phadke V, Haynes LJ, Calfee DP, Yin MT. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J Infect. 2012;65(1):80–7.
Durakovic N, Radojcic V, Boban A, et al. Efficacy and safety of colistin in the treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa in patients with hematologic malignancy: a matched pair analysis. Intern Med. 2011;50(9):1009–13.
Gauthier TP, Wolowich WR, Reddy A, Cano E, Abbo L, Smith LB. Incidence and predictors of nephrotoxicity associated with intravenous colistin in overweight and obese patients. Antimicrob Agents Chemother. 2012;56(5):2392–6.
Montero M, Horcajada JP, Sorlí L, et al. Effectiveness and safety of colistin for the treatment of multidrug-resistant Pseudomonas aeruginosa infections. Infection. 2009;37(5):461–5.
Kim J, Lee KH, Yoo S, Pai H. Clinical characteristics and risk factors of colistin-induced nephrotoxicity. Int J Antimicrob Agents. 2009;34(5):434–8.
Cheng CY, Sheng WH, Wang JT, Chen YC, Chang SC. Safety and efficacy of intravenous colistin (colistin methanesulphonate) for severe multidrug-resistant gram-negative bacterial infections. Int J Antimicrob Agents. 2010;35(3):297–300.
Paul M, Bishara J, Levcovich A, et al. Effectiveness and safety of colistin: prospective comparative cohort study. J Antimicrob Chemother. 2010;65(5):1019–27.
Deryke CA, Crawford AJ, Uddin N, Wallace MR. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob Agents Chemother. 2010;54(10):4503–5.
Bassetti M, Repetto E, Righi E, et al. Colistin and rifampicin in the treatment of multidrug-resistant Acinetobacter baumannii infections. J Antimicrob Chemother. 2008;61(2):417–20.
Kallel H, Hergafi L, Bahloul M, et al. Safety and efficacy of colistin compared with imipenem in the treatment of ventilator-associated pneumonia: a matched case-control study. Intensive Care Med. 2007;33(7):1162–7.
Garnacho-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ, et al. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36(9):1111–8.
Levin AS, Barone AA, Penço J, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28(5):1008–11.
Falagas ME, Rafailidis PI, Ioannidou E, et al. Colistin therapy for microbiologically documented multidrug-resistant gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35(2):194–9.
Hachem RY, Chemaly RF, Ahmar CA, et al. Colistin is effective in treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa in cancer patients. Antimicrob Agents Chemother. 2007;51(6):1905–11.
Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J Antimicrob Chemother. 2008;61(6):1369–75.
Gounden R, Bamford C, van Zyl-Smit R, Cohen K, Maartens G. Safety and effectiveness of colistin compared with tobramycin for multi-drug resistant Acinetobacter baumannii infections. BMC Infect Dis. 2009;9:26.
Lim SK, Lee SO, Choi SH, et al. The outcomes of using colistin for treating multidrug resistant Acinetobacter species bloodstream infections. J Korean Med Sci. 2011;26(3):325–31.
Kvitko CH, Rigatto MH, Moro AL, Zavascki AP. Polymyxin B versus other antimicrobials for the treatment of Pseudomonas aeruginosa bacteraemia. J Antimicrob Chemother. 2011;66(1):175–9.
Ouderkirk JP, Nord JA, Turett GS, Kislak JW. Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrob Agents Chemother. 2003;47(8):2659–62.
Mostardeiro MM, Pereira CA, Marra AR, Pestana JO, Camargo LF. Nephrotoxicity and efficacy assessment of polymyxin use in 92 transplant patients. Antimicrob Agents Chemother. 2013;57(3):1442–6.
Hartzell JD, Neff R, Ake J, Wortmann G. et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48(12):1724–8.
Ko Hj, Jeon Mh, Choo Ej, et al. Early acute kidney injury is a risk factor that predicts mortality in patients treated with colistin. Nephron Clin Pract. 2011;117(3):284–8.
Kellum JA, Bellomo R, Ronco C. Definition and classification of acute kidney injury. Nephron Clin Pract. 2008;109(4):182–7.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4.
Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292(2):670–86.
Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–95.
Yousef JM, Chen G, Hill PA, Nation RL, Li J. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother. 2011;55(9):4044–9.
Ozyilmaz E, Ebinc FA, Derici U, et al. Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine? Intensive Care Med. 2010;37(1):141–6.
Yousef JM, Chen G, Hill PA, Nation RL, Li J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother. 2012;67(2):452–9.
Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–41.
Desai TK, Tsang TK. Aminoglycoside nephrotoxicity in obstructive jaundice. Am J Med. 1988;85(1):47–50.
Oliveira JF, Silva JA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53(7):2887–91.
Rattanaumpawan P, Lorsutthitham J, Ungprasert P, Angkasekwinai N, Thamlikitkul V. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by gram-negative bacteria. J Antimicrob Chemother. 2010;65(12):2645–9.
Betrosian AP, Frantzeskaki F, Xanthaki A, Douzinas EE. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect. 2008;56(6):432–6.
Reina R, Estenssoro E, Sáenz G, et al. Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med. 2005;31(8):1058–65.
Rios FG, Luna CM, Maskin B, et al. Ventilator-associated pneumonia due to colistin susceptible-only microorganisms. Eur Respir J. 2007;30(2):307–13.
Conflict of interest
-
1.
“The English translation of manuscript was made by Gilead Pharmaceutical Company”.
-
2.
The study have been presented in Congress (EKMUD 2013 Bilimsel Platformu, Antalya, Turkey). Pfizer Pharmaceutical Company have made payment for Congress attendance and travel fees of Bahadır Ceylan.
-
3.
The study have been presented in Congress (ID Week 2013, San Francisco, USA). Pfizer and Novartis Pharmaceutical Company have made payment for Congress attendance and travel fees of Turan Aslan and Yasemin Akkoyunlu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceylan, B., Taniş, M., Akkoyunlu, M. et al. Variables determining the development of colistin-associated renal impairment. Wien Klin Wochenschr 128 (Suppl 8), 614–619 (2016). https://doi.org/10.1007/s00508-015-0773-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-015-0773-z