Key message
Pathways for assimilates.
Abstract
During their life cycle, plants alternate between a haploid stage, the gametophyte, and a diploid stage, the sporophyte. In higher plants, meiosis generates the gametophyte deeply embedded in the maternal tissue of the flower. The megaspore mother cell undergoes meiosis, and then, the surviving megaspore of the four megaspores produced undergoes mitotic divisions and finally gives rise to the female gametophyte, consisting of the egg cell, two synergids, the central cell, which due to the fusion of two nuclei is diploid (double haploid) in Arabidopsis and most angiosperms and the antipods, whose number is not fixed and varies significantly between species (Yadegari and Drews in Plant Cell 16(Suppl):S133–S141, 2004). The maternal tissues that harbor the female gametophyte and the female gametophyte are referred to as the ovule (Fig. 1). Double fertilization of the egg cell and the central cell by the two generative nuclei of the pollen leads to the diploid embryo and the endosperm, respectively (Hamamura et al. in Curr Opin Plant Biol 15:70–77, 2012). Upon fertilization, the ovule is referred to as the seed. Seeds combine two purposes: to harbor storage compounds for use by the embryo upon germination and to protect the embryo until the correct conditions for germination are encountered. As a consequence, seeds are the plant tissue that is of highest nutritional value and the human diet, by a considerable amount, consists of seeds or seed-derived products. Amino acids are of special interest, because plants serve as the main source for the so-called essential amino acids, that animals cannot synthesize de novo and are therefore often a limiting factor for human growth and development (WHO in Protein and amino acid requirements in human nutrition. WHO technical report series, WHO, Geneva, 2007). The plant embryo needs amino acids for general protein synthesis, and additionally they are used to synthesize storage proteins in the seeds of certain plants, e.g., legumes as a resource to support the growth of the seedling after germination. The support of the embryo depends on transport processes that occur between the mother plant and the seed tissues including the embryo. In this review, we will focus on the processes of unloading amino acids from the phloem and their post-phloem transport. We will further highlight similarities between amino acid transport and the transport of the main assimilate and osmolyte, sucrose. Finally, we will discuss similarities and differences between different plant species in terms of structural aspects but for the molecular aspects we are almost exclusively focusing on Arabidopsis.

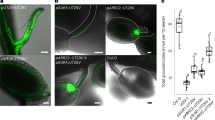
Vascularization of the Arabidopsis ovule and seed. Plants expressing ER-localized mCherry under control of the companion cell-specific SUC2 promoter and ER-localized GFP under control of the sieve element marker PD1 as described (Müller et al. 2015) are shown to visualize the phloem in the funiculus and the chalazal regions. a Overview over an ovule. FG: female gametophyte. b A magnification of the region marked by a square in panel a. c Overview over a seed. ES: endosperm; E: embryo. d A magnification of the region marked by a square in panel c. The arrows in b and d point to the terminal companion cell and arrowheads to terminal sieve elements

Similar content being viewed by others
References
Baud S, Boutin JP, Miquel M, Lepiniec L, Rochat C (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 40:151–160
Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273:948–950
Besnard J, Pratelli R, Zhao C, Sonawala U, Collakova E, Pilot G, Okumoto S (2016) UMAMIT14 is an amino acid exporter involved in phloem unloading in Arabidopsis roots. J Exp Bot 67:6385–6397
Borg S, Brinch-Pedersen H, Tauris B, Holm PB (2009) Iron transport, deposition and bioavailability in the wheat and barley grain. Plant Soil 325:15–24
Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, Chermak D, Antony G, White FF, Somerville SC, Mudgett MB, Frommer WB (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532
Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335:207–211
Chen LQ, Lin IW, Qu XQ, Sosso D, McFarlane HE, Londono A, Samuels AL, Frommer WB (2015) A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell 27:607–619
Coen O, Fiume E, Xu W, De Vos D, Lu J, Pechoux C, Lepiniec L, Magnani E (2017) Developmental patterning of the sub-epidermal integument cell layer in Arabidopsis seeds. Development 144:1490–1497
Ferro M, Salvi D, Rivière-Rolland H, Vermat T, Seigneurin-Berny D, Grunwald D, Garin J, Joyard J, Rolland N (2002) Integral membrane proteins of the chloroplast envelope: identification and subcellular localization of new transporters. Proc Natl Acad Sci USA 99:11487–11492
Feuerstein A, Niedermeier M, Bauer K, Engelmann S, Hoth S, Stadler R, Sauer N (2010) Expression of the AtSUC1 gene in the female gametophyte, and ecotype-specific expression differences in male reproductive organs. Plant Biol (Stuttg) 12(Suppl 1):105–114
Frommer WB, Hummel S, Riesmeier JW (1993) Expression cloning in yeast of a cDNA encoding a broad specificity amino acid permease from Arabidopsis thaliana. Proc Natl Acad Sci USA 90:5944–5948
Grallath S, Weimar T, Meyer A, Gumy C, Suter-Grotemeyer M, Neuhaus JM, Rentsch D (2005) The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol 137:117–126
Hamamura Y, Nagahara S, Higashiyama T (2012) Double fertilization on the move. Curr Opin Plant Biol 15:70–77
Hammes UZ, Nielsen E, Honaas LA, Taylor CG, Schachtman DP (2006) AtCAT6, a sink-tissue-localized transporter for essential amino acids in Arabidopsis. Plant J 48:414–426
Han X, Hyun TK, Zhang M, Kumar R, Koh EJ, Kang BH, Lucas WJ, Kim JY (2014) Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev Cell 28:132–146
Hill LM, Morley-Smith ER, Rawsthorne S (2003) Metabolism of sugars in the endosperm of developing seeds of oilseed rape. Plant Physiol 131:228–236
Kim I, Cho E, Crawford K, Hempel FD, Zambryski PC (2005a) Cell-to-cell movement of GFP during embryogenesis and early seedling development in Arabidopsis. Proc Natl Acad Sci USA 102:2227–2231
Kim I, Kobayashi K, Cho E, Zambryski PC (2005b) Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc Natl Acad Sci USA 102:11945–11950
Kleffmann T, Russenberger D, von Zychlinski A, Christopher W, Sjolander K, Gruissem W, Baginsky S (2004) The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr Biol 14:354–362
Knoblauch M, Knoblauch J, Mullendore DL, Savage JA, Babst BA, Beecher SD, Dodgen AC, Jensen KH, Holbrook NM (2016) Testing the Münch hypothesis of long distance phloem transport in plants. eLife 5:e15341
Ladwig F, Stahl M, Ludewig U, Hirner AA, Hammes UZ, Stadler R, Harter K, Koch W (2012) Siliques are red1 from Arabidopsis acts as a bidirectional amino acid transporter that is crucial for the amino acid homeostasis of siliques. Plant Physiol 158:1643–1655
Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55:341–372
Lucas WJ, Ding B, van der Schoot C (1994) Plasmodesmata and the supracellular nature of plants. New Phytol 125:435–476
Morley-Smith ER, Pike MJ, Findlay K, Kockenberger W, Hill LM, Smith AM, Rawsthorne S (2008) The transport of sugars to developing embryos is not via the bulk endosperm in oilseed rape seeds. Plant Physiol 147:2121–2130
Müller B (2016) Characterization of UmamiTs: amino acid transporters involved in amino acid cycling, phloem unloading and the supply of symplasmically isolated sink tissues. PhD thesis, Regensburg University, Regensburg
Müller B, Fastner A, Karmann J, Mansch V, Hoffmann T, Schwab W, Suter-Grotemeyer M, Rentsch D, Truernit E, Ladwig F, Bleckmann A, Dresselhaus T, Hammes UZ (2015) Amino acid export in developing Arabidopsis seeds depends on UmamiT facilitators. Curr Biol 25:3126–3131
Offler CE, Patrick JW (1984) Cellular structures, plasma membrane surface areas and plasmodesmatal frequencies of seed coats of Phaseolus vulgaris L. in relation to photosynthate transfer. Aust J Plant Physiol 11:79–99
Offler CE, Nerlich SM, Patrick JW (1989) Pathway of photosynthate transfer in the developing seed of Vicia faba L. Transfer in relation to seed anatomy. J Exp Bot 40:769–780
Pommerrenig B, Popko J, Heilmann M, Schulmeister S, Dietel K, Schmitt B, Stadler R, Feussner I, Sauer N (2013) SUCROSE TRANSPORTER 5 supplies Arabidopsis embryos with biotin and affects triacylglycerol accumulation. Plant J 73:392–404
Ranocha P, Denance N, Vanholme R, Freydier A, Martinez Y, Hoffmann L, Kohler L, Pouzet C, Renou JP, Sundberg B, Boerjan W, Goffner D (2010) Walls are thin 1 (WAT1), an Arabidopsis homolog of Medicago truncatula NODULIN21, is a tonoplast-localized protein required for secondary wall formation in fibers. Plant J 63:469–483
Ranocha P, Dima O, Nagy R, Felten J, Corratge-Faillie C, Novak O, Morreel K, Lacombe B, Martinez Y, Pfrunder S, Jin X, Renou JP, Thibaud JB, Ljung K, Fischer U, Martinoia E, Boerjan W, Goffner D (2013) Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat Commun 4:2625
Rentsch D, Schmidt S, Tegeder M (2007) Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett 581:2281–2289
Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11:4705–4713
Roberts AG, Cruz SS, Roberts IM, Prior D, Turgeon R, Oparka KJ (1997) Phloem unloading in sink leaves of Nicotiana benthamiana: comparison of a fluorescent solute with a fluorescent virus. Plant Cell 9:1381–1396
Rolletschek H, Hosein F, Miranda M, Heim U, Gotz KP, Schlereth A, Borisjuk L, Saalbach I, Wobus U, Weber H (2005) Ectopic expression of an amino acid transporter (VfAAP1) in seeds of Vicia narbonensis and pea increases storage proteins. Plant Physiol 137:1236–1249
Ross-Elliott TJ, Jensen KH, Haaning KS, Wager BM, Knoblauch J, Howell AH, Mullendore DL, Monteith AG, Paultre D, Yan D, Otero S, Bourdon M, Sager R, Lee JY, Helariutta Y, Knoblauch M, Oparka KJ (2017) Phloem unloading in Arabidopsis roots is convective and regulated by the phloem-pole pericycle. eLife 6:e24125
Sanders A, Collier R, Trethewy A, Gould G, Sieker R, Tegeder M (2009) AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J 59:540–552
Sauer N, Stadler R (1993) A sink-specific H+/monosaccharide co-transporter from Nicotiana tabacum: cloning and heterologous expression in baker’s yeast. Plant J 4:601–610
Schneitz K, Hulskamp M, Pruitt RE (1995) Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. Plant J 7:731–749
Schwacke R, Schneider A, van der Graaff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flugge U-I, Kunze R (2003) ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol 131:16–26
Sosso D, Luo D, Li QB, Sasse J, Yang J, Gendrot G, Suzuki M, Koch KE, McCarty DR, Chourey PS, Rogowsky PM, Ross-Ibarra J, Yang B, Frommer WB (2015) Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat Genet 47:1489–1493
Stadler R, Lauterbach C, Sauer N (2005) Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol 139:701–712
Tan Q, Zhang L, Grant J, Cooper P, Tegeder M (2010) Increased phloem transport of S-methylmethionine positively affects sulfur and nitrogen metabolism and seed development in pea plants. Plant Physiol 154:1886–1896
Tegeder M, Hammes UZ (2017) The way out and in: phloem loading and unloading of amino acids. Curr Opin Plant Biol 43:16–21
Tegeder M, Masclaux-Daubresse C (2018) Source and sink mechanisms of nitrogen transport and use. New Phytol 217:35–53
Tilsner J, Nicolas W, Rosado A, Bayer EM (2016) Staying tight: plasmodesmal membrane contact sites and the control of cell-to-cell connectivity in plants. Annu Rev Plant Biol 67:337–364
Werner D, Gerlitz N, Stadler R (2011) A dual switch in phloem unloading during ovule development in Arabidopsis. Protoplasma 248:225–235
Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H, Wobus U (2000) Sucrose transport into barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J 21:455–467
WHO (2007) Protein and amino acid requirements in human nutrition. WHO technical report series. WHO, Geneva
Yadegari R, Drews GN (2004) Female gametophyte development. Plant Cell 16(Suppl):S133–S141
Yeung EC, Meinke DW (1993) Embryogenesis in angiosperms: development of the suspensor. Plant Cell 5:1371–1381
Zhang LY, Peng YB, Pelleschi-Travier S, Fan Y, Lu YF, Lu YM, Gao XP, Shen YY, Delrot S, Zhang DP (2004) Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol 135:574–586
Zhang W-H, Zhou Y, Dibley KE, Tyerman SD, Furbank RT, Patrick JW (2007) Nutrient loading of developing seeds. Funct Plant Biol 34:314–331
Zhang LZ, Garneau MG, Majumdar R, Grant J, Tegeder M (2015) Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J 81:134–146
Acknowledgements
We are grateful for funding through DFG SFB924 TPA08. We thank two anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Lepiniec, H. North, G. Ingram.
A contribution to the special issue ‘Seed Biology’.
Rights and permissions
About this article
Cite this article
Karmann, J., Müller, B. & Hammes, U.Z. The long and winding road: transport pathways for amino acids in Arabidopsis seeds. Plant Reprod 31, 253–261 (2018). https://doi.org/10.1007/s00497-018-0334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-018-0334-5