Abstract
Key message
Pine tree species exhibit significant levels of phenotypic variation in the investment in defences, which can be correlated with life-history traits, geographical affiliations and climate.
Abstract
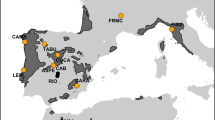
Understanding the ecological and environmental correlates of tree defences has value for understanding forest susceptibility to pests and pathogens in a time of global change. In the present work, we assessed how life-history attributes and biogeography are related to chemical defences of pine trees in Palaearctic and Nearctic forests. We studied adult trees of ten pine species in forests of Portugal and Eastern North America. We measured total phenols (using gallic acid as a standard) and condensed tannins (as catechin hydrate equivalents) in the phloem of pine branches. Pine trees in forests of Eastern North America presented higher levels of total phenolic content in their phloem tissues than pine trees in forests in Portugal. Higher values of precipitation were correlated with higher phenolic content and higher temperatures were associated with higher levels of condensed tannins. A few life-history traits—the maximum height reached by each pine species, the age at which they start reproducing, and the size of seeds—were positively related with defences. The present work points to interactions between life-history attributes, climate, and geographic location as predictors of defensive investment in pines. The results are useful for understanding differences within and among pine forests in susceptibility to pests and pathogens.



Similar content being viewed by others
Data availability
Data and code will be available upon request.
References
Adams JM, Woodward FI (1989) Patterns in tree species richness as a test of the glacial extinction hypothesis. Nature 339(6227):699–701
Anstett DN, Nunes KA, Baskett C, Kotanen PM (2016) Sources of controversy surrounding latitudinal patterns in herbivory and defense. Trends Ecol Evol 31(10):789–802
Aukema JE, McCullough DG, Von Holle B, Liebhold AM, Britton K, Frankel SJ (2010) Historical accumulation of nonindigenous forest pests in the continental United States. Bioscience 60(11):886–897
Awada T, Radoglou K, Fotelli MN, Constantinidou HIA (2003) Ecophysiology of seedlings of three Mediterranean pine species in contrasting light regimes. Tree Physiol 23(1):33–41
Barbehenn RV, Constabel PC (2011) Tannins in plant–herbivore interactions. Phytochemistry 72(13):1551–1565
Brasier CM (1991) Ophiostoma novo-ulmi sp. nov causative agent of current Dutch elm disease pandemics. Mycopathologia 115(3):151–161
Carrillo-Gavilán A, Moreira X, Zas R, Gonzalez-Voyer A, Vilà M, Sampedro L (2015) Phylogenetic and biogeographical patterns in defensive strategies and quantitative allocation to chemical defences in Palaearctic and Nearctic pine trees. J Biogeogr 42(4):684–693
Coley PD, Aide TM (1991) A comparison of herbivory and plant defenses in temperate and tropical broad–leaved forests. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant–animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp 25–49
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230(4728):895–899
De Luis M, Novak K, Čufar K, Raventós J (2009) Size mediated climate–growth relationships in Pinus halepensis and Pinus pinea. Trees 23(5):1065–1073
Endara MJ, Coley PD (2011) The resource availability hypothesis revisited: a meta-analysis. Funct Ecol 25(2):389–398
Feeny P (1976) Plant apparency and chemical defense. In: Wallace JW, Mansell RL (eds) Recent advances in phytochemistry, vol 10. Plenum Press, New York, pp 1–40
Firmino PN, Calvão T, Ayres MP, Pimentel CS (2017) Monochamus galloprovincialis and Bursaphelenchus xylophilus life history in an area severely affected by pine wilt disease: Implications for forest management. Forest Ecol Manag 389:105–115
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167(2):353–376
Friedenberg NA, Whited BM, Slone DH, Martinson SJ, Ayres MP (2007) Differential impacts of the southern pine beetle, Dendroctonus frontalis, on Pinus palustris and Pinus taeda. Can J for Res 37(8):1427–1437
Garnas JR, Houston DR, Ayres MP, Evans C (2012) Disease ontogeny overshadows effects of climate and species interactions on population dynamics in a non-native forest disease complex. Ecography 35(5):412–421
Gandhi KJ, Herms DA (2010) Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions 12(2):389–405
He T, Pausas JG, Belcher CM, Schwilk DW, Lamont BB (2012) Fire-adapted traits of Pinus arose in the fiery cretaceous. New Phytol 194(3):751–759
Heil M, Baumann B, Andary C, Linsenmair KE, McKey D (2002) Extraction and quantification of condensed tannins as a measure of plant anti-herbivore defence? Revisit Old Probl Naturwissenschaften 89(11):519–524
Hicke JA, Allen CD, Dietze DAR, MC, Hall RJ, Hogg EH, Kashian DM, Moore D, Raffa KF, Sturrock RN, Vogelmann J, (2012) Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob Change Biol 18(1):7–34
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25(15):1965–1978
Holopainen JK, Virjamo V, Ghimire RP, Blande JD, Julkunen-Tiitto R, Kivimäenpää M (2018) Climate change effects on secondary compounds of forest trees in the northern hemisphere. Front Plant Sci 9:1445
Huntley B (1993) Species-richness in north-temperate zone forests. J Biogeogr 20:163–180
Karban R (2011) The ecology and evolution of induced resistance against herbivores. Funct Ecol 25(2):339–347
Keeley JE, Zedler PH (1998) Evolution of life histories in Pinus. In: Richardson DM (ed) Ecology and biogeography of Pinus. Cambridge University Press, Cambridge, UK, pp 219–250
Keeley JE (2012) Ecology and evolution of pine life histories. Ann for Sci 69(4):445–453
Kempel A, Schädlerb M, Chrobocka T, Fischera M, van Kleunena M (2011) Tradeoffs associated with constitutive and induced plant resistance against herbivory. Proc Natl Acad Sci USA 108(14):5685–5689
Lavery PB, Mead DJ (1998) Pinus radiata: a narrow endemic from North America takes on the world. In: Richardson DM (ed) Ecology and biogeography of Pinus. Cambridge University Press, Cambridge, UK, pp 432–449
Lev-Yadun S, Holopainen JK (2009) Why red-dominated autumn leaves in America and yellow-dominated autumn leaves in Northern Europe? New Phytol 183(3):506–512
Liebhold AM, McCullough DG, Blackburn LM, Frankel SJ, Von Holle B, Aukema JE (2013) A highly aggregated geographical distribution of forest pest invasions in the USA. Divers Distrib 19(9):1208–1216
Liebhold AM, Yamanaka T, Roques A, Augustin S, Chown SL, Brockerhoff EG, Pyšek P (2016) Global compositional variation among native and non-native regional insect assemblages emphasizes the importance of pathways. Biol Invasions 18(4):893–905
Loehle C (1988) Tree life history strategies: the role of defenses. Can J for Res 18(2):209–222
Lovett GM, Weiss M, Liebhold AM, Holmes TP, Leung B, Lambert KF, Orwig DA, Campbell FT, Rosenthal J, McCullough DG, Wildova R, Ayres MP, Canham CD, Foster DR, LaDeau SL, Weldy T (2016) Non-native forest insects and pathogens in the United States: impacts and policy options. Ecol Appl 26(5):1437–1455
Marquis RJ, Ricklefs RE, Abdala-Roberts L (2012) Testing the low latitude/high defense hypothesis for broad-leaved tree species. Oecologia 169(3):811–820
Milgroom MG, Wang K, Zhou Y, Lipari SE, Kaneko S (1996) Intercontinental population structure of the chestnut blight fungus Cryphonectria Parasitica. Mycologia 88(2):179–190
Moles AT, Westoby M (2006) Seed size and plant strategy across the whole life cycle. Oikos 113(1):91–105
Moreira X, Mooney KA, Rasmann S, Petry WK, Carrillo-Gavillan A, Zas R, Sampedro L (2014) Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecol Lett 17(5):537–546
Pimentel CS, Ayres MP, Vallery E, Young C, Streett DA (2014) Geographical variation in seasonality and life history of pine sawyer beetles Monochamus spp: its relation with phoresy by the pinewood nematode Bursaphelenchus xylophilus. Agric Forest Entomol 16(2):196–206
Pimentel CS, Gonçalves EV, Firmino PN, Calvão T, Fonseca L, Abrantes I, Correia O, Máguas C (2017) Differences in constitutive and inducible defences in pine species determining susceptibility to pinewood nematode. Plant Pathol 66(1):131–139
Platt WJ, Evans GW, Rathbun SL (1988) The population dynamics of a long-lived conifer (Pinus palustris). Ame Nat 131(4):491–525
Proches S, Wilson JRU, Richardson DM, Rejmanek M (2012) Native and naturalized range size in Pinus: relative importance of biogeography, introduction effort and species traits. Glob Ecol Biogeogr 21(5):513–523
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. UK, Cambridge University Press, Cambridge
Raffa KF, Mason CJ, Bonello P, Cook S, Erbilgin N, Keefover-Ring K, Klutsch JG, Villari C, Townsend PA (2017) Defence syndromes in lodgepole–whitebark pine ecosystems relate to degree of historical exposure to mountain pine beetles. Plant Cell Environ 40(9):1791–1806
Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164(S3):S143–S164
Richardson DM, Rundel PW (1998) Ecology and biogeography of Pinus: an introduction. In: Richardson DM (ed) Ecology and biogeography of Pinus. UK, Cambridge University Press, Cambridge, pp 3–46
Samuelson LJ, Stokes TA, Butnor JR, Johnsen KH, Gonzalez-Benecke CA, Anderson P, Jackson J, Ferrari L, Martin TA, Wendell PC, Cropper WP Jr (2014) Ecosystem carbon stocks in Pinus palustris forests. Can J for Res 44(5):476–486
Santini A, Ghelardini L, De Pace C, Desprez-Loustau ML, Capretti P, Chandelier A, Cech T, Chira D, Diamandis S, Gaitniekis T, Hantula J, Holdenrieder O, Jankovsky L, Jung T, Jurc D, Kirisits T, Kunca A, Lygis V, Malecka M, Marcais B, Schmitz S, Schumacher J, Solheim H, Solla A, Szabo I, Tsopelas P, Vannini A, Vettraino AM, Webber J, Woodward S, Stenlid J (2013) Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol 197(1):238–250
Schafstall N, Kuosmanen N, Fettig CJ, Knižek M, Clear JL (2020) Late Glacial and Holocene records of tree-killing conifer bark beetles in Europe and North America: implications for forest disturbance dynamics. Holocene 30(6):847–857
Schulz AN, Mech AM, Allen CR, Ayres MP, Gandhi KJ, Gurevitch J, Havill NP, Herms DA, Hufbauer RA, Liebhold AM, Raffa KF, Raupp MJ, Thomas KA, Tobin PC, Marsico TD (2020) The impact is in the details: evaluating a standardized protocol and scale for determining non-native insect impact. NeoBiota 55:61–83
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am Soc Enol Viticulture 16(3):144–158
Stamp N (2003) Out of the quagmire of plant defense hypotheses. Q Rev Biol 78(1):23–55
Sturrock RN, Frankel SJ, Brown AV, Hennon PE, Kliejunas JT, Lewis KJ, Worrall JJ, Woods AJ (2011) Climate change and forest diseases. Plant Pathol 60(1):133–149
Tapias R, Climent J, Pardos JA, Gil L (2004) Life histories of Mediterranean pines. Plant Pathol 171(1–2):53–68
Tomback DF, Linhart YB (1990) The evolution of bird-dispersed pines. Evol Ecol 4(3):185–219
Zas R, Moreira X, Sampedro L (2011) Tolerance and induced resistance in a native and an exotic pine species: relevant traits for invasion ecology. J Ecol 99(6):1316–1326
Acknowledgements
We are grateful to Carla Rodrigues for the discussion and implementation of the methods for chemical analysis, and Ana Tomás and Arborea (Vinhais, Portugal) for all the support they provided during field work in the northeast of Portugal. We also thank Erwin Beck for constructive comments and suggestions that greatly improved the paper.
Funding
This study was funded by the Portuguese Foundation for Science and Technology (FCT) through: the projects PTDC/AGR-CFL/098869/2008 and PTDC/ASP-SIL/29774/2017 (also funded by the programmes COMPETE 2020 and Portugal 2020 from the European Regional Development Fund FEDER); the grant SFRH/BPD/46995/2008 and the contract DL57/2016/CP1382/CT0009 conceded to C.S. Pimentel: funding conceded to the Forest Research Centre—UIDB/00239/2020); and to CENSE—UIDB/04085/2020. Additional support to the work in the USA was provided by a grant provided by the Luso-American Development Foundation (FLAD) in support to the FCT project PTDC/AGR-CFL/098869/2008.
Author information
Authors and Affiliations
Contributions
CSP and MPA conceptualized the ideas behind the work; CSP, EVG and MPA collected the samples in the Field; CM, OC and EVG conceptualized the work on the secondary metabolites, while EVG did all the analytical work; JC and TC collected and analysed the data on life-history, geographical and environmental variables of the different pine species; CSP analysed the data and wrote the manuscript; All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Beck .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pimentel, C.S., Gonçalves, E.V., Campôa, J. et al. Ecogeographical determinants of investment in chemical defences in pines. Trees 37, 361–372 (2023). https://doi.org/10.1007/s00468-022-02354-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-022-02354-5