Abstract
Key message
Walnut production in California depends on the performance of three clonal hybrid rootstocks, and this study provides evidence that roots of genotype RX1 exhibit unique properties to cope with water stress induced by drought.
Abstract
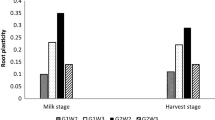
Three hybrid genotypes RX1 (Juglans microcarpa × J. regia), Vlach and VX211 (both J. hindsii × J. regia) are the most commonly used rootstocks for walnut production in California. These rootstocks provide various levels of disease resistance, but their performance under drought is unknown. Based on our findings on xylem stress physiology of native walnut species, we hypothesize that hybrid genotypes originating from wild species native to drier habitats will exhibit superior root performance under drought stress. Using a whole-plant experimental approach, we evaluated root and canopy physiological characteristics of 2-year-old tree saplings of RX1, Vlach and VX211 under two soil moisture treatments (‘control’ 70–90% and ‘drought’ 20–40% w/w soil moisture). In control saplings, root biomass was twofold smaller in RX1 as compared to VX211, but root system hydraulic conductance (Kro, predominantly cell-to-cell pathway) was more than 50% greater in RX1 and Vlach as compared to VX211. The relatively low Kro of VX211 was related to a larger number of root cortical cell layers and endodermis development. In drought-stressed saplings, root biomass was reduced by 27% (P < 0.05) in VX211 but no significant reduction in root biomass was detected in RX1 and Vlach. Maintenance of root biomass under drought in RX1 and Vlach was associated with an 80% decrease in Kro, a threefold increase in leaf intrinsic water-use efficiency, and maintenance of leaf turgor as compared to control conditions. Drought-induced reductions in Kro were linked to the formation of a multiseriate root endodermis in all three genotypes.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Albacete A, Martinez-Andjar C, Martinez-Perez A, Thompson AJ, Dodd IC, Perez-Alfocea F (2015) Unravelling rootstock s scion interactions to improve food security. J Exp Bot 66:2211–2226
Aleta N, Vilanova A, Diaz R, Voltas J (2009) Genetic variation for carbon isotope composition in Juglans regia L.: relationships with growth, phenology and climate origin. Ann For Sci 66:1–11
Aroca R, Porcel R, Ruiz-Lozano JM (2012) Regulation of root water uptake under abiotic stress conditions. J Exp Bot 63:43–57
Barrios-Masias FH, Knipfer T, Walker MA, McElrone AJ (2018) Differences in hydraulic traits of grapevine rootstocks are not conferred to a common Vitis vinifera scion. Funct Plant Biol 46:228–235
Behrooz A, Vahdati K, Rejali F, Lotfi M, Sarikhani S, Leslie C (2019) Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. HortScience 54:1087–1092
Blum A (2017) Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ 40:4–10
Brown L, Ramos D, Uriu K, Marrangoni B (1977) Walnut moisture stress studies. Report to the California Walnut Board
Buchner R, Fulton A, Gilles C, Lampinen B, Shackel K, Metcalf S, Little C, Prichard T, Schwankl L (2008) Effects of regulated deficit irrigation on walnut (Juglans regia) grafted on Northern California Black (Juglans hindsii) or Paradox Rootstock. Acta Hort 792:141–146
Buzo T, McKenna J, Kaku S, Anwar SA, McKenry MV (2009) VX211, a vigorous new walnut hybrid clone with nematode tolerance and a useful resistance mechanism. J Nematol 41:211–216
Christensen L (2003) Rootstock selection. In: Christensen PL, Dokoozlian KN, Walker AM, Wolpert JA (eds) Wine grape varieties in California. University of California Agricultural and Natural Resources Publication, Oakland, pp 12–15
Comas L, Becker S, Cruz VMV, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4:442
Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2002) Improving intrinsic water-used efficiency and crop yield. Crop Sci 42:122–131
Contador ML et al (2015) Root growth dynamics linked to above-ground growth in walnut (Juglans regia). Ann Bot 116:49–60
Cuneo IF, Knipfer T, Brodersen CR, McElrone AJ (2016) Mechanical failure of fine root cortical cells initiates plant hydraulic decline during drought. Plant Physiol 172:1669–1678
DeJong TM, Johnson RS, Doyle TF, Basile B (2004) Growth, yield and physiological behavior of size-controlling peach rootstocks developed in California. Acta Hortic 658:449–455
DeJong TM, Lindsay G, Almehdi A, Johnson RS, Day KR (2014) Performance and physiology of the Controller series of peach rootstocks. Acta Hortic 1058:523–529
Delmas M, Delzon S, Cochard H, Herbette S (2018) Is there variability for xylem vulnerability to cavitation in walnut tree cultivars and species (Juglans spp.)? Hortic Sci 53:132–137
Dudley SA (1996) Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution 50:92–102
Enstone DE, Peterson CA, Ma F (2003) Root endodermis and exodermis: structure, function and responses to the environment. J Plant Growth Regul 21:335–351
Famula RA, Richards JH, Famula TR, Neale DB (2019) Association genetics of carbon isotope discrimination and leaf morphology in a breeding populations of Juglans regia L. Tree Genet Genomes 15:1–13
Farquhar GD, Leary MHO, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Gambetta GA, Knipfer T, Fricke W, McElrone AJ (2017) Aquaporins and root water uptake. In: Tyerman SD (ed) Plant aquaporins. Springer, Cham
Goldhamer DA, Beede R, Sibbett S, DeJong TM, Ramos D, Phene RC, Doyle J (1987) Second year effects of deficit irrigation on walnut tree performance. Report to the California Walnut Board
Hasey J (2016) Selecting the right clonal rootstock for managing soil and pest problems. University of California Agriculture and Natural Resources. http://www.sacvalleyorchards.com/blog/walnuts-blog/selecting-the-right-clonal-rootstock-for-managing-soil-and-pest-problems/. Accessed 2019
Heschel MS, Riginos C (2005) Mechanisms of selection for drought stress tolerance and avoidance in Impatiens Capensis (Balsaminaceae). Am J Bot 92:37–44
Jerszurki D, Couvreur V, Maxwell T, de Carvalho Ramos Silva L, Matsumoto N, Shackel K, Moretti de Souza JL, Hopmans J (2017) Impact of root growth and hydraulic conductance on canopy carbon-water relations of young walnut trees (Juglans regia L.) under drought. Sci Hortic 266:342–352
Johnson R, Cody BA (2015) California agricultural production and irrigated water use. Congressional Research Service, Washington
Jones MM, Turner NC (1978) Osmotic adjustment in leaves of Sorghum in response to water deficits. Plant Physiol 61:122–126
Knipfer T, Fricke W (2010) Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare). New Phytol 187:159–170
Knipfer T, Fricke W (2011) Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.). J Exp Bot 62:717–733
Knipfer T, Brodersen CR, Zedan A, Kluepfel DA, McElrone AJ (2015) Patterns of drought-induced embolism formation and spread in living walnut saplings visualized using X-ray microtomography. Tree Physiol 35:744–755
Knipfer T, Barrios-Masias F, Cuneo IF, Bouda M, Albuquerque CP, Brodersen CR, Kluepfel DA, McElrone AJ (2018) Variations in xylem embolism susceptibility under drought between intact saplings of three walnut species. Tree Physiol 38:1180–1192
Kramer PJ, Boyer JS (1995) Water relations of plant and soil. Academic Press, San Diego
Maurel C, Verducq L, Luu DT, Santoni V (2007) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
McDowell N, Pockman WT, Allen CD et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Meng D, Fricke W (2017) Changes in root hydraulic conductivity facilitate the overall hydraulic response of rice (Oryza sativa L.) cultivars to salt and osmotic stress. Plant Physiol Biochem 113:64–77
Meyer CJ, James LS, Peterson CA (2009) Environmental effects on the maturation of the endodermis and multiseriate exodermis of Iris germanica roots. Ann Bot 103:687–702
North GB, Nobel PS (1991) Changes in hydraulic conductivity and anatomy caused by drying and rewetting roots of Agave deserti (Agavaceae). Am J Bot 78:906–915
Passioura JB (2002) Soil conditions and plant growth. Plant, Cell Environ 25:311–318
Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. J Exp Biol 58:113–117
Rosati A, Metcalf S, Buchner R, Fulton A, Lampinen B (2006) Tree water status and gas exchange in walnut under drought, high temperature and vapour pressure deficit. J Hortic Sci Biotechnol 81:415–420
Schreiber L, Franke RB (2011) Endodermis and exodermis in roots. In: eLS. Wiley, Chichester
Seibt U, Rajabi A, Griffiths Berry JA (2008) Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155:441–454
Steudle E, Peterson CA (1998) How does water get through roots? J Exp Bot 49:775–788
Tomos D (2000) The plant cell pressure probe. Biotechnol Lett 22:437–442
Vahdati K, Lotfi N (2009) Abiotic stress tolerance in plants with emphasizing on drought and salinity stresses in walnut—chapter 10. In: Vahdati K (ed) Abiotic stress—plant responses and applications in agriculture, IntechOpen. https://www.intechopen.com/books/abiotic-stress-plant-responses-and-applications-in-agriculture
Vahdati K, Lotfi N, Kholdebarin B, Hassani D, Amiri R, Mozaffari MR, Leslie C (2009) Screening for drought-tolerant genotypes of Persian Walnuts (Juglans regia L.) during seed germination. HortScience 44:1815–1819
Williams LE, Araujo FJ (2002) Correlations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera. J Am Hortic Sci 127:448–454
Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33:740–749
Acknowledgements
We would like to thank Sierra Gold Nursery (Yuba City, CA, USA) for providing the plant material and Kenneth Shackel (UC Davis) for his helpful comments on this manuscript. We also want to thank Jackson Grom, Molly Clemens, Sarah Tracy, Elisabeth Forrestel and Idan Reingwirtz for their help with data collection.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by deMicco.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Knipfer, T., Reyes, C., Momayyezi, M. et al. A comparative study on physiological responses to drought in walnut genotypes (RX1, Vlach, VX211) commercially available as rootstocks. Trees 34, 665–678 (2020). https://doi.org/10.1007/s00468-019-01947-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-019-01947-x