Abstract
Key message
The analysis of the growth behaviour, wood anatomical, and eco-physiological traits of two Bougainvillea genotypes, trained to two shapes and subjected to different irrigation regimes, fully supports the idea that structural properties interact with cultural practices (i.e., training) in determining the adaptive capability of plants, hence their productivity and survival.
Abstract
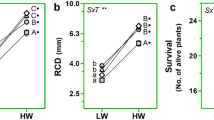
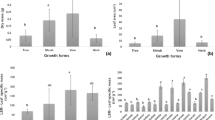
Bougainvillea species are cultivated for landscaping in the arid Mediterranean region. In Bougainvillea spp., secondary xylem and phloem are formed by successive cambia leading to a peculiar stem anatomy which could favour water storage and plant adaptation under drought conditions. To achieve sustainable production of Bougainvillea ornamental shrubs, it is crucial to understand how different genotypes respond to deficit irrigation and how cultural practices, such as canopy training, may interact with morpho-anatomical traits in modifying the plants’ ability to withstand water deficit. A greenhouse experiment was conducted to determine the effects of deficit irrigation on plant growth, ecophysiology, and branch anatomy in two Bougainvillea genotypes [B. × buttiana ‘Rosenka’, B. ‘Lindleyana’ (=B. ‘Aurantiaca’)] trained to globe and pyramid shapes. Irrigation treatments were based on daily evapotranspiration (ET): control (C, 100 % ET) or deficit irrigation (DI, 25 % ET). The two genotypes exhibited morphological adaptations to cope with water deficit, including reductions in dry weight, leaf number, and lamina size. In both genotypes, the DI-induced increase in stomatal resistance was accompanied by a decrease in stomata size. Water deficit triggered adjustments in wood anatomical functional traits, also depending on canopy shape and genotype, favouring either water conduction efficiency or safety against embolism. The occurrence of a safer hydraulic system in the pyramid-trained plants suggests a better control of water transport, thus supporting better growth performance under DI conditions compared to globe-trained plants. Such differences, induced by different canopy-shape trainings, should be considered in the management of DI.





Similar content being viewed by others
References
Álvarez S, Navarro A, Bañón S, Sánchez-Blanco MJ (2009) Regulated deficit irrigation in potted dianthus plants: effects of severe and moderate water stress on growth and physiological responses. Sci Hort 122:579–585
Álvarez S, Navarro A, Nicolás E, Sánchez-Blanco MJ (2011) Transpiration, photosynthetic responses, tissue water relations and dry mass partitioning in Callistemon plants during drought conditions. Sci Hort 129:306–312
Álvarez S, Sánchez-Blanco MJ (2013) Changes in growth rate, root morphology and water use efficiency of potted Callistemon citrinus plants in response to different levels of water deficit. Sci Hort 156:54–62
Beeckman H (2016) Wood anatomy and trait-based ecology. IAWA J 37:127–151
Beeson RC Jr (2004) Modeling actual evapotranspiration of Ligustrum japonicum from rooted cuttings to commercially marketable plants in 12 L black polyethylene containers. Acta Hort 664:71–77
Bernal M, Estiarte M, Peñuelas J (2011) Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora. Plant Biol 13:252–257
Braidwood L, Breuer C, Sugimoto K (2013) Mybody is a cage: mechanisms and modulation of plant cell growth. New Phytol 201:388–402
Brodersen CR, McElrone AJ, Choat B, Lee EF, Shackel KA, Matthews MA (2013) In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiol 161:1820–1829
Brodribb TJ (2009) Xylem hydraulic physiology: the functional backbone of terrestrial plant productivity. Plant Sci 177:245–251
Cameron R, Harrison-Murray R, Fordham M, Wilkinson S, Davies W, Atkinson C, Else M (2008) Regulated irrigation of woody ornamentals to improve plant quality and precondition against drought stress. Ann Appl Biol 153:49–61
Cameron RWF, Harrison-Murray RS, Atkinson CJ, Judd HL (2006) Regulated deficit irrigation: a means to control growth in woody ornamentals. J Hort Sci Biotechnol 81:435–443
Carlquist S (1989) Adaptive wood anatomy of chaparral shrubs. In: Keely JE (ed) The California chaparral: paradigms re-examined. Los Angeles Country Museum of Natural History Contributions, Los Angeles, pp 25–35
Carlquist S (2007) Successive cambia revisited: ontogeny, histology, diversity, and functional significance. J Torrey Bot Soc 134:301–332
Carlquist S (2012) How wood evolves: a new synthesis. Botany 90:901–940
Carter JL, White DA (2009) Plasticity in the Huber value contributes to homeostasis in leaf water relations of a mallee Eucalypt with variation to groundwater depth. Tree Physiol 29:1407–1418
Casa R, Rouphael Y (2014) Effects of partial root-zone drying irrigation on yield, fruit quality, and water-use efficiency in processing tomato. J Hort Sci Biotechnol 89:389–396
Cella Pizarro L, Bisigato AJ (2010) Allocation of biomass and photoassimilates in juvenile plants of six Patagonian species in response to five water supply regimes. Ann Bot 106(2):297–307
Chaves MM, Pereira JS, Moroco J, Rodrigues ML, Ricardo CPP, Osório ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field. Photosynthesis and growth. Ann Bot 89:907–916
Chew S (2010) Anatomical features of Bougainvillea (Nyctaginaceae). Stud Undergrad Res Guelph 4:72–78
Cirillo C, Rouphael Y, Caputo R, Raimondi G, De Pascale S (2014) The influence of deficit irrigation on growth, ornamental quality and water use efficiency of three potted Bougainvillea genotypes grown in two shapes. Hort Sci 49(10):1284–1291
Cirillo C, Rouphael Y, Caputo R, Raimondi G, De Pascale S (2015) Water stress responses of five potted Bougainvillea genotypes. Acta Hort 1107:203–208
Corcuera L, Camarero JJ, Gil-Pelegrín E (2004) Effects of a severe drought on growth and wood anatomical properties of Quercus faginea. IAWA J 25:185–204
De Micco V, Aronne G (2007) Combination of histochemistry and auto-fluorescence for the identification of lignin distribution in cell walls. Biotech Histochem 82:209–216
De Micco V, Aronne G (2009) Seasonal dimorphism in wood anatomy of the Mediterranean Cistus incanus L. subsp. incanus. Trees 23:981–989
De Micco V, Aronne G (2012a) Morpho-anatomical traits for plant adaptation to drought. In: Aroca R (ed) Plant responses to drought stress: from morphological to molecular features. Springer, Berlin, pp 37–62
De Micco V, Aronne G (2012b) Anatomy and lignin characterization of twigs in the chaparral shrub Rhamnus californica. IAWA J 33:151–162
De Micco V, Aronne G, Baas P (2008) Wood anatomy and hydraulic architecture of stems and twigs of some Mediterranean trees and shrubs along a mesic-xeric gradient. Trees 22:643–655
De Micco V, Campelo F, de Luis M, Bräuning A, Grabner M, Battipaglia G, Cherubini P (2016) Intra-annual density fluctuations in tree rings: how, when, where, and why? IAWA J 37:232–259
Drake PL, Froend RH, Franks PJ (2013) Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot 64:495–505
Egea G, Nortes PA, González-Real MM, Baille A, Domingo A (2010) Agronomic response and water productivity of almond trees under contrasted deficit irrigation regimes. Agric Water Manag 97:171–181
Franco JA, Martínez-Sánchez JJ, Fernández JA, Bañón S (2006) Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J Hort Sci Biotechnol 81:3–17
Fukuzawa K (1992) Ultraviolet microscopy. In: Lin SY, Dence CW (eds) Methods in lignin chemistry. Springer, Berlin, pp 110–131
Grant OM, Davies MJ, Longbottom H, Harrison-Murray R (2012) Evapotranspiration of container ornamental shrubs: modelling crop-specific factors for a diverse range of crops. Irrig Sci 30:1–12
Hacke UG, Sperry JS, Wheeler JK, Castro L (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol 26(6):689–701
Hansen CW, Petersen KK (2004) Reduced nutrient and water availability to Hibiscus rosa-sinensis ‘Cairo Red’ as a method to regulate growth and improve post-production quality. Eur J Hort Sci 69:159–166
Hsiao TC, Steduto P, Fereres E (2007) A systematic and quantitative approach to improve water use efficiency in agricolture. Irrig Sci 25:209–231
ISMEA (2015) Il mercato dei prodotti florovivaistici. Ottobre-Novembre 2014, Gennaio-Maggio 2015. http://www.ismeaservizi.it. Accessed 27 Oct 2015
Jensen WA (1962) Botanical histochemistry. Principle and practice. Freeman WH & Company, San Francisco CA
Kobayashi KD, McConnell J, Griffis J (2007) Bougainvillea, ornamentals and flowers. Published by the College of Tropical Agriculture and Human Resources (CTAHR). http://www.ctahr.hawaii.edu/oc/freepubs/pdf/OF-38.pdf. Accessed 13 Mar 2014
Ludlow MM (1980) Adaptive significance of stomatal responses to water stress. In: Turner NC, Kramer P-J (eds) Adaptation of plants to water and high temperature stress. Wiley, New York, pp 123–128
McCulloh KA, Sperry JS (2005) Patterns in hydraulic architecture and their implications for transport efficiency. Tree Physiol 25:257–267
Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR (2009) Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct Ecol 23:922–930
Morris H, Plavcová L, Cvecko P, Fichtler E, Gillingham MFA, Martínez-Cabrera HI, McGlinn DJ, Wheeler E, Zheng J, Ziemińska K, Jansen S (2016) A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytol 209:1553–1565
Niu G, Rodriguez DS, Mackay W (2008) Growth and physiological response to drought stress in four oleander clones. J Am Soc Hort Sci 133:188–196
Olson ME, Anfodillo T, Rosell JA, Petit G, Crivellaro A, Isnard S, Léon-Gómez C, Alvarado-Cárdenas LO, Castorena M (2014) Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecol Lett 17:988–997
Pfautsch S, Harbusch M, Wesolowski A, Smith R, Macfarlane C, Tjoelker MG, Reich PB, Adams MA (2016) Climate determines vascular traits in the ecologically diverse genus Eucalyptus. Ecol Lett 19:240–248
Robert EMR, Schmitz N, Boeren I, Driessens T, Herremans K, De Mey J, Van de Casteele E, Beeckman H, Koedam N (2011) Successive cambia: a developmental oddity or an adaptive structure? PLoS ONE 6:e16558
Robert EMR, Schmitz N, Copini P, Gerkema E, Vergeldt FJ, Windt CW, Beeckman H, Koedam N, Van As H (2014) Visualization of the stem water content of two genera with secondary phloem produced by successive cambia through Magnetic Resonance Imaging (MRI). J Plant Hydraulics 1:e-0006
Rouphael Y, Cardarelli M, Colla G, Rea E (2008) Yield, mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. Hort Sci 43:730–736
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, New York
Sánchez-Blanco MJ, Álvarez S, Navarro A, Bañón S (2009) Changes in leaf water relations, gas exchange, growth and flowering quality in potted geranium plants irrigated with different water regimes. Plant Physiol 166:467–476
Scheiber SM, Beeson RC Jr (2007) Landscape growth and aesthetic quality of coleus managed with irrigation deficits. Hort Technol 17:561–566
Schuetz M, Smith R, Ellis B (2013) Xylem tissue specification, patterning, and differentiation mechanisms. J Exp Bot 64:11–31
Silber A, Levi M, Cohen M, David N, Shtaynmetz Y, Assouline S (2007) Response of Leucadendron ‘Safari Sunset’ to regulated deficit irrigation: effects of stress timing on growth and yield quality. Agric Water Manag 87:162–170
Souza JP, Prado CHBA, Albino ALS, Damascos MA, Souza GM (2011) Network analysis of tree crowns distinguishes functional groups of Cerrado species. Plant Ecol 212:11–19
Toscano S, Scuderi D, Giuffrida F, Romano D (2014) Responses of Mediterranean ornamental shrubs to drought stress and recovery. Sci Hort 178:145–153
Tyree MT, Alexander JD (1993) Hydraulic conductivity of branch junctions in three temperate tree species. Trees 7:156–159
Ugolini F, Tognetti R, Bussotti F, Raschi A, Ennos AR (2014) Wood hydraulic and mechanical properties induced by low water availability on two ornamental species Photinia × fraseri var. Red Robin and Viburnum opulus L. Urban For Urban Gree 13:158–165
Vasquez-Cooz I, Meyer RW (2002) A differential staining method to identify lignified and unlignified tissues. Biotech Histochem 77:277–282
Von Arx G, Kueffer C, Fonti P (2013) Quantifying plasticity in vessel grouping—added value from the image analysis tool ROXAS. IAWA J 34:433–445
Wheeler EA (1986) Vessels per square millimetre or vessel groups per square millimetre? IAWA J 7:73–74
Wheeler JK, Sperry JS, Hacke UG, Huang N (2005) Inter-vessel pitting and cavitation in woody Rosaceae and other vesseled plants: a basis for a safety vs. efficiency trade-off in xylem transport. Plant Cell Environ 28:800–812
Willaume M, Lauri PE, Sinoquet H (2004) Light interception in apple trees influenced by canopy architecture manipulation. Trees 18:705–713
WWAP (World Water Assessment Programme) (2014) The united nations world water development report 2014: water and energy. UNESCO, Paris
Zimmermann MH (1978) Hydraulic architecture of some diffuse-porous trees. Can J Bot 56:2286–2295
Zimmermann MH (2002) Xylem structure and the ascent of sap. Springer, Berlin
Acknowledgments
This work was funded by the Italian Ministry of Agricultural, Food, and Forestry Policies (MiPAAF) under the project ‘‘Tecnologie di filiera per il controllo della tolleranza a stress idrico in Bougainvillea’’ (D.M. 11053/7643/09 of 7 May 2009). The authors wish to thank Giampaolo Raimondi for his technical support. The authors thank Mark Walters for editing the final draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by K. Masake.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cirillo, C., De Micco, V., Rouphael, Y. et al. Morpho-anatomical and physiological traits of two Bougainvillea genotypes trained to two shapes under deficit irrigation. Trees 31, 173–187 (2017). https://doi.org/10.1007/s00468-016-1466-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1466-6