Abstract
Background
Compared with the general population, the immune response to COVID-19 mRNA vaccines is lower in adult kidney transplant recipients (KTRs). However, data is limited for pediatric KTRs. In this study, we aimed to assess humoral and cellular immune responses to the COVID-19 mRNA vaccine in pediatric KTRs.
Methods
This multicenter, prospective, case–control study included 63 KTRs (37 male, aged 12–21 years), 19 dialysis patients, and 19 controls. Humoral (anti-SARS-CoV2 IgG, neutralizing Ab (nAb)) and cellular (interferon-gamma release assay (IGRA)) immune responses were assessed at least one month after two doses of BNT162b2 mRNA vaccine.
Results
Among COVID-19 naïve KTRs (n = 46), 76.1% tested positive for anti-SARS-CoV-2 IgG, 54.3% for nAb, and 63% for IGRA. Serum levels of anti-SARS-CoV-2 IgG and nAb activity were significantly lower in KTRs compared to dialysis and control groups (p < 0.05 for all). Seropositivity in KTRs was independently associated with shorter transplant duration (p = 0.005), and higher eGFR (p = 0.007). IGRA titer was significantly lower than dialysis patients (p = 0.009). Twenty (43.4%) KTRs were positive for all immune parameters. Only four of 11 seronegative KTRs were IGRA-positive. COVID-19 recovered KTRs had significantly higher anti-SARS-CoV-2 IgG and nAb activity levels than COVID-19 naïve KTRs (p = 0.018 and p = 0.007, respectively).
Conclusions
The humoral and cellular immune responses to SARS-CoV-2 mRNA BNT162b2 vaccine are lower in pediatric KTRs compared to dialysis patients. Further prospective studies are required to demonstrate the clinical efficacy of the mRNA vaccine in KTRs.
This prospective study was registered in ClinicalTrials.gov (NCT05465863, registered retrospectively at 20.07.2022).
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information.
Similar content being viewed by others
Introduction
Coronavirus 2019 (COVID-19) infection is associated with higher morbidity and mortality in adult patients on dialysis and kidney transplant recipients (KTRs) [1,2,3,4]. Pediatric KTRs develop asymptomatic or mild COVID-19 disease with a favorable outcome [5]. An increased risk of subclinical acute kidney injury (AKI) is associated with mild to moderate COVID-19 in children; however, less is known about the transplanted kidney [6]. Given KTRs are immunosuppressed and vulnerable to infection and SARS-CoV-2 appears to have an affinity for the kidney, vaccination of this population is important.
It is well established that vaccine response (to attenuated, conjugated, or recombinant) is lower in pediatric dialysis patients and KTRs when compared with the general population [7, 8]. This is attributed to uremia and immunosuppressant medications [9]. The new mRNA vaccine technology is being used worldwide, including in children and adolescents during the pandemic. Studies have demonstrated a lower immune response to the new SARS-CoV-2 mRNA vaccine in adult KTRs [10,11,12,13,14,15,16,17]. However, there are limited data on the immune response elicited by the vaccine in children and adolescents with kidney replacement therapy [18, 19].
The aim of this study was to investigate both humoral and cellular immune responses to two-doses of BNT162b2 mRNA COVID-19 vaccine in pediatric KTRs compared with dialysis patients and healthy controls. The humoral immune response was assessed using anti-SARS-CoV-2 immunoglobulin G (anti-SARS-CoV-2 IgG) and SARS-CoV-2 neutralizing antibody (nAb). The cellular immune response was assessed using the SARS-CoV-2-specific interferon-ɣ-release assay (IGRA).
Material and methods
Study design
This prospective, multicenter case–control study was conducted with the participation of five pediatric nephrology centers in Istanbul between September 2021 and March 2022. The centers were asked to report all dialysis and kidney transplant patients between the ages of 12 and 21 years to be vaccinated against COVID-19. Patients were informed about the vaccine according to local vaccination schedule and requested to make an appointment for vaccination. The BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech®) was administered intramuscularly into the deltoid region of all participants who agreed to participate in the study. At least one month after the second vaccine dose, serum and whole blood samples were collected from all patients and controls to analyze the humoral and cellular immune response to the vaccine. All samples were stored at –20 °C until assayed. SARS-CoV-2 PCR test results were collected retrospectively to determine natural SARS-CoV-2 infection. Patients with a history of positive SARS-CoV-2 PCR were defined as “COVID-19 recovered,” and the remaining were defined as “COVID-19 naïve.” The control group consisted of 19 age- and gender-comparable healthy children. See Fig. 1 for the flow diagram of the study design. All patients were recommended a third dose of the vaccine, but only 13 of 63 KTRs received a third dose.
Assessment of immune response to SARS-CoV-2 vaccine
Humoral response
Humoral immune response was assessed with anti-SARS-CoV-2 IgG quantification (anti-SARS-CoV-2 IgG) and neutralization test (nAb activity). Anti-SARS-CoV-2 IgG antibody titers, which prevent the binding of SARS-CoV-2 S1/RBD region to ACE2 receptors, were determined by SARS-CoV-2 QuantiVac ELISA (IgG) (Euroimmun AG, Lübeck, Germany). Neutralization capacities of these antibodies were also determined by SARS-CoV-2 NeutraLISA (Euroimmun AG, Lübeck, Germany). Antibody titers were obtained in relative units/mL (RU/mL) (1 RU/mL * 3.2 = 1 Binding Antibody Unit per mL (BAU/mL)). Antibody titer values below 8 RU/mL (25.6 BAU/mL) were interpreted as “seronegative,” and values above 11 RU/mL (35.2 BAU/mL) were interpreted as “seropositive,” according to manufacturer’s guidelines. Serum samples that exceeded assay measuring range (> 120 RU/ml) were diluted by a 1:10 factor and tested again to obtain more accurate results. Neutralizing antibody responses were assessed as percent inhibition (%IH). Percent inhibition values below 20% were considered “nAb-negative,” and values above 35% were considered “nAb-positive,” according to manufacturer’s guidelines.
Cellular immune response
Cellular immune response was assessed with IGRA. A specific stimulation of T-cells by the spike protein of SARS-CoV-2 was performed using the Quan-T-Cell SARS-CoV-2 (Euroimmun AG, Lübeck, Germany) to determine the amount of IFN-γ released by immune cells. IFN-y responses were then measured using the Quan-T-Cell ELISA (Euroimmun AG, Lübeck, Germany) Interferon Gamma Release Assay. Results were obtained in milli-international units per milliliter (mIU/mL) in accordance with the manufacturer’s instructions. Values below 100 mIU/mL were interpreted as “IGRA-negative,” and values above 200 mIU/mL were interpreted as “IGRA-positive.”
Statistical analyses
The Statistical Package for Social Sciences (SPSS) for Windows version 20.0 (SPSS, IBM Corporation, Chicago) was used for analysis. GraphPad Prism version 9.4.0 (GraphPad Software, San Diego, CA) was used for figures. Normality of the data were tested with the Kolmogorov–Smirnov test. Continuous data were expressed as mean ± standard deviation (SD) in the case of a normal distribution and analyzed with the Student t-test or one-way ANOVA test. In the case of a non-normal distribution, data were expressed as the median (interquartile range, 25th; 75th percentiles) and analyzed using the Mann–Whitney U test or the Kruskal–Wallis test. Categorical variables were expressed as n (%) and analyzed with Chi-Square test. Bonferroni correction was applied when appropriate. The correlation between anti-SARS-CoV-2 IgG, nAb, and IGRA levels were analyzed using the Spearman correlation test. Variables with a p value of < 0.1 were analyzed with backward multivariate logistic regression analysis to determine the independent predictors of anti-SARS-CoV-2 IgG and nAb positivity. A two tailed p value < 0.05 was defined as significant.
Results
A total of 63 KTRs, 19 dialysis patients (15 HD and 4 PD), and 19 healthy controls were included in the study (Fig. 1 and Table 1). The median time between second vaccine dose and the assessment of immune response was 8 weeks (7;14 weeks) in the KTRs. The difference was not statistically significant from dialysis and control groups. There was no statistically significant difference in age, gender, or SARS-CoV-2 PCR positivity between the three groups (Table 1). Forty-nine of the KTRs (78%) were on standard triple immunosuppressive therapy (prednisolone, tacrolimus, and mycophenolate mofetil/mycophenolic acid (MMF/MPA)), five were on triple treatment with prednisolone, mTORi, and MMF/MPA, and four were on tacrolimus/cyclosporin and MMF/MPA. Fifty-five KTRs (82.5%) received a kidney from a living donor. Acute rejection history was present in six KTRs; none of which occurred within 6 months of the study. Median time posttransplantation was 77 months with all participants having received a transplant greater than 12 months prior to the study. A total of 17 KTRs had a history of COVID-19 before vaccinations; the median time between COVID-19 and evaluation of immune response to vaccine was 47 weeks.
Immune response to SARS-CoV-2 vaccine in COVID-19 naïve study population
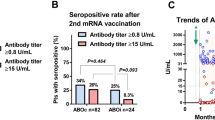
The immune responses to the vaccine in COVID-19 naïve study populations are given in Table 1 and Fig. 2. KTRs had significantly lower anti-SARS-CoV-2 IgG titer levels than both dialysis (p = 0.020) and control (p = 0.023) groups (Fig. 2A). KTRs also had lower anti-SARS-CoV-2 IgG positivity (76.1%) but the difference did not reach statistical significance, either for dialysis (100%) or control (100%) groups (p = 0.099 for both, Fig. 2B). Furthermore, KTRs had significantly lower nAb activity levels than both dialysis (p = 0.006) and control (p = 0.002) groups (Fig. 2C). KTRs had also lower nAb positivity (54.3%) than dialysis (81.8%) and control (100%) groups, but the difference was statistically significant only between KTRs and controls (p = 0.004) (Fig. 2D).
Comparisons of humoral and cellular immune responses between kidney transplant recipients (KTRs), dialysis patients, and control subjects, among COVID-19 naïve study population. A Anti-SARS-CoV2 IgG titer, B anti-SARS-CoV2 IgG seropositivity rate, C neutralizing antibody (nAb) activity, D nAb positivity rate, E interferon gamma release assay (IGRA) titer, and F IGRA positivity rate. Only the differences between groups with a p value < 0.10 are shown in the figure
The prevalence of IGRA positivity in KTRs, dialysis, and control groups were 63% (29/46), 100% (11/11), and 81.8% (9/11), respectively. KTRs also had lower IGRA titer levels compared to other two groups. However, the difference was statistically significant only between KTRs and dialysis group for both positivity (p = 0.024) and titer level (p = 0.009) (Fig. 2E and F).
None of the humoral (anti-SARS-CoV-2 IgG, nAb) and cellular (IGRA) immune parameters demonstrated statistical significance between dialysis and control groups (Table 1).
Factors affecting immune response to SARS-CoV-2 vaccine in COVID-19 naïve KTRs
KTRs with a positive anti-SARS-CoV-2 IgG had significantly shorter time on transplantation (p = 0.005) and higher eGFR (p = 0.007) compared to seronegative KTRs (Table 2). Three of the 11 seronegative KTRs had a history of rituximab due to acute rejection, while none of the seropositive group had such a history (p = 0.012). These three KTRs had an eGFR < 50 ml/min/1.73 m2 and one of them had hypogammaglobulinemia. In multivariate logistic regression analysis, only shorter time on transplantation and higher eGFR were independently associated with a positive anti-SARS-CoV-2 IgG (ß: −0.586, OR: 0.961, 95% CI: 0.924–0.998 and ß: 0.079, OR: 0.1.082, 95% CI: 1.014–1.155, respectively). KTRs with a positive nAb activity had higher levels of tacrolimus dose, but the difference did not reach statistical significance (p = 0.063, Table 2). There was no statistical significance between IGRA-positive (n = 29) and IGRA-negative KTRs (n = 17) in terms of clinical or laboratory parameters (Table 2).
The relationship between humoral and cellular immunity among COVID-19 naïve KTRs
The distribution of humoral and cellular immune responses in the COVID-19 naïve KTRs is shown in Fig. 3. Out of 35 anti-SARS-CoV-2 IgG seropositive KTRs, 10 (28.5%) were nAb-negative. Four out of 11 anti-SARS-CoV-2 IgG seronegative KTRs were IGRA-positive. A complete immune response (positive anti-SARS-CoV-2 IgG, nAb, and IGRA) was observed in 20 KTRs (43.4%), whereas 7/46 KTRs (15.2%) showed no immune response at all. All immune parameters—anti-SARS-CoV-2 IgG, nAb activity, and IGRA titer levels—were significantly correlated with each other (p < 0.001 for all, Fig. 4).
Comparison of immune response to SARS-CoV-2 between COVID-19 naïve and recovered KTRs
COVID-19 recovered KTRs had significantly higher titers of both anti-SARS-COV-2 IgG and nAb compared to COVID-19 naïve KTRs (p = 0.018 and p = 0.007 respectively, Table 1). In terms of positivity, COVID-19 recovered KTRs had significantly higher nAb positivity (100% vs. 54.3% respectively, p = 0.003), but anti-SARS-CoV-2 IgG did not differ significantly (94.1% vs. 76.1% respectively, p = 0.155). Neither IGRA titer level nor IGRA positivity differed significantly according to COVID-19 history (p > 0.05 for both).
During the study period, six out of 63 KTRs had COVID-19 after two doses of SARS-CoV-2 vaccine. Two of them had no humoral or cellular immune response to the vaccine. None of them experienced a severe disease needing hospitalization. Thirteen of 63 KTRs received a third dose of the vaccine. Only one of four anti-SARS-COV-2 IgG-seronegative KTRs and two of five IGRA-negative KTRs had a positive result after the third dose of the vaccine.
Discussion
In this prospective multicenter study, both humoral and cellular immune responses to the two doses of BNT162b2 mRNA COVID-19 vaccine were assessed in pediatric KTRs and compared with pediatric dialysis patients and healthy controls. The main findings of our study were that COVID-19 naïve KTRs have significantly lower levels of anti-SARS-CoV-2 IgG titer and nAb activity compared to both dialysis and control groups, demonstrating lower vaccine-induced humoral immunity among KTRs. Shorter time on transplantation and higher eGFR were independently associated with anti-SARS-CoV-2 seropositivity in the KTRs. Furthermore, COVID-19 naïve KTRs had significantly lower IGRA levels than dialysis patients. They also demonstrated a trend toward lower IGRA levels than controls, but the difference did not reach statistical significance. COVID-19 recovered KTRs had significantly higher anti-SARS-CoV-2 IgG titer and nAb activity levels compared to COVID-19 naïve KTRs, but IGRA titers did not differ significantly. These findings demonstrated the booster effect of natural SARS-CoV-2 infection on humoral immunity, but not on cellular immunity. Dialysis patients demonstrated similar humoral and cellular immune response to the SARS-CoV-2 mRNA vaccine compared to the healthy individuals, which is similar to data in adult cohorts [15, 16, 20].
The reported prevalence of a positive humoral immune response to SARS-CoV-2 mRNA vaccines varies widely in adult KTRs due to differences in study protocols, established cut-off values, and sensitivity of different assays. The prevalence of seroconversion in KTRs has been reported to range from 36 to 63%, which is significantly lower compared to both CKD, dialysis, and healthy individuals [10,11,12,13,14,15,16]. Younger age, lower MMF dose, low tacrolimus trough level, and higher eGFR have been reported to be associated with improved humoral immune response [10,11,12, 15,16,17], whereas shorter time on transplantation, especially post-transplant first year, has been associated with a negative humoral immune response [15, 16].
There are few studies conducted in pediatric KTRs. Haskin et al. [18] demonstrated a 63% seroconversion rate in 38 adolescents and young adults with a mean age of 18 years after two doses of BNT162b2 mRNA vaccine. The authors reported that seroconverted KTRs had a significantly lower use of rituximab and a longer time after the second vaccine dose compared to seronegative KTRs. Crane et al. [19] have reported a 52% seroconversion rate after two doses of BNT162b2 mRNA vaccine in 25 adolescents with a median age of 19 years. The authors reported a higher number of KTRs on MMF and higher doses of MMF use in the non-responders. In the present study, the seroconversion rate was 76.1% in COVID-19 naïve KTRs. This higher prevalence of seroconversion compared to previous studies among adolescents and adults may be partly explained by younger age of our cohort or by the different assays used in these studies. Consistent with the previous studies, anti-SARS-CoV-2 IgG seropositivity in our cohort was associated with higher eGFR in KTRs. Lower eGFR does not explain lower immunity against SARS-CoV-2 vaccination as dialysis patients with much lower eGFR had significantly higher anti-SARS-CoV-2 IgG than KTRs. Three of the eleven anti-SARS-CoV-2 seronegative KTRs were given rituximab due to acute rejection. These patients had low eGFR and one of them had hypogammaglobulinemia. Although rituximab or acute rejection history did not remain in the regression analysis, they may have an effect on eGFR and seropositivity association. The seronegative group was small; larger cohorts are needed to assess this association. In contrast to the published reports, shorter time on transplantation was associated with seropositivity in the present study. However, it is important to note that in our cohort none of the KTRs had a shorter transplant duration than 12 months. Although the seroconversion rate seems higher than previous studies, COVID-19 naïve KTRs had still significantly lower anti-SARS-CoV-2 IgG titers compared to both dialysis and control groups.
Neutralizing antibody activity indicates the functional antibodies that can inhibit SARS-CoV-2 infection; in other words, it represents the clinical efficacy of vaccine-induced measured antibodies [21]. Lower nAb titers have been reported in adult KTRs compared with healthy controls [16, 22], but pediatric data is not yet available. The prevalence of nAb positivity has been reported to range from 31 to 65.8% in adult studies including all KTRs. On the other hand, in the studies including only seropositive adult KTRs, this prevalence has been reported as high as 79.3%, which is still lower than in healthy controls [13, 16, 22]. In our cohort, COVID-19 naïve KTRs had significantly lower nAb activity compared to both dialysis and control groups. The prevalence of nAb positivity was also significantly lower in COVID-19 naïve KTRs than controls (54.3% vs. 100%). In line with the literature, there was a strong correlation between the titer of anti-SARS-CoV-2 IgG and nAb activity [16]. The frequency of a negative nAb among anti-SARS-CoV-2 IgG seropositive KTRs was about 30% in the present study, which has been reported as 10% by Pedersen et al. [22]. These findings demonstrate that not only seropositivity but also titer levels of anti-SARS-CoV-2 IgG are important to predict protection from COVID-19 in KTRs.
It is known that repeated vaccination may not elicit a humoral response but a cellular immune response. The prevalence of a positive cellular immune response to an mRNA vaccine has been reported to range between 16.2 and 60% in adult KTRs [15, 16, 23, 24]. The current study was the first to investigate the cellular immune response in pediatric KTRs after SARS-CoV-2 vaccination. The prevalence of a positive cellular response was 63% in COVID-19 naïve KTRs, which was quite low compared with both dialysis patients (100%) and healthy controls (81.8%). Both the positivity and titer levels of IGRA were significantly lower compared to dialysis patients, but not from controls. However, the number of COVID-19 naïve healthy controls was low. Four of eleven seronegative KTRs had positive cellular immunity. We could not detect any clinical or laboratory factors affecting cellular immune response.
The effect of natural COVID-19 on immunity in vaccinated KTRs was assessed in adults by Magicova et al. [25]. They demonstrated a higher prevalence of seroconversion after two doses of BNT162b2 or Moderna mRNA-1273 vaccine, in KTRs with a COVID-19 history, than COVID-19 naïve vaccinated KTRs (97% vs. 40%). In our study, COVID-19 recovered KTRs had significantly higher titers of anti-SARS-CoV-2 IgG and nAb activity compared to COVID-19 naïve KTRs. These results demonstrate the booster effect of natural infection on humoral immunity. Although the IGRA positivity was higher in COVID-19 recovered KTRs than COVID-19 naïve KTRs (100% vs. 81%), the difference did not reach statistical significance. Magicova et al. [25] also demonstrated a better cellular immune response in previously infected, vaccinated adult KTRs compared with naïve vaccinated KTRs (90% vs. 9%).
This study demonstrated a trend toward an improved cellular immune response at higher anti-SARS-CoV-2 IgG titers. The seroconversion rate appears to be high, but a complete immune response, i.e., positive nAb and cellular immune response in addition to seroconversion, was present in about 40% of the KTRs. This may be indicative of the lower clinical efficacy of the SARS-CoV-2 mRNA vaccine in pediatric KTRs. These results suggest that booster vaccination and/or possibly an increase in vaccine dose is needed, similar to HBV vaccination in CKD patients. In our cohort, only 13 KTRs received a third dose of the vaccine during the study period. One out of four anti-SARS-CoV-2 IgG seronegative and two out of five IGRA-negative KTRs were positive after a third dose of vaccine. It is difficult to draw significance from this, given the small sample size. Nevertheless, results from the adult studies demonstrate an enhanced humoral and cellular immune response after the third dose of mRNA vaccine in KTRs [23, 26].
Our study has several limitations. Sampling was planned after 4 weeks following the second vaccine dose; however, the delay in study approval resulted in heterogenous timing of blood sampling with a median 8 weeks in KTRs. Nevertheless, timing was not significantly different from dialysis or control groups. Secondly, due to this delay in the study start, we missed the prevaccination sampling to measure anti-SARS-CoV-2 IgG to determine natural SARS-CoV-2 infection. Therefore, we defined natural SARS-CoV-2 infection with a previously positive PCR test, which may result in asymptomatic cases being missed. Lastly, the number of dialysis and control groups was small. Although KTRs had lower IGRA levels and positivity than controls, the difference was not statistically significant. This finding can be explained by the small sample size for controls and the sensitivity of the assay. The strength of our study is that we assessed not only seroconversion but also detailed immune analyses, including SARS-CoV-2-specific nAb and cellular immune responses to mRNA vaccination, in a relatively high number of KTRs.
In conclusion, the humoral and cellular immune response after two doses of SARS-CoV-2 mRNA vaccination appears to be better in pediatric KTRs than in adult KTRs, whereas the immune response is still lower compared to healthy children. In particular, KTRs with longer transplant duration and lower eGFR have a lower humoral immune response, whereas natural SARS-CoV-2 infection has a booster effect on the humoral immune response. Although seroconversion prevalence appears to be high, only about 40% of the KTRs have both a positive nAb and T-cell immune response in addition to seroconversion, which may demonstrate the need for booster doses or an increase in vaccine dose. Further prospective studies are required to demonstrate the clinical efficacy of the SARS-CoV-2 mRNA vaccine-induced immune response in KTRs.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Chung EYM, Palmer SC, Natale P, Krishnan A, Cooper TE, Saglimbene VM, Ruospo M, Au E, Jayanti S, Liang A, Jie Deng DJ, Chui J, Higgins GY, Tong A, Wong G, Teixeira-Pinto A, Hodson EM, Craig JC, Strippoli GFM (2021) Incidence and outcomes of COVID-19 in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis 78:804–815. https://doi.org/10.1053/j.ajkd.2021.07.003
Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sanchez-Alvarez JE, Garneata L, Collart F, Hemmelder MH, Ambuhl P, Kerschbaum J, Legeai C, Del Pino YPMD, Mircescu G, Mazzoleni L, Hoekstra T, Winzeler R, Mayer G, Stel VS, Wanner C, Zoccali C, Massy ZA (2020) Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 98:1540–1548. https://doi.org/10.1016/j.kint.2020.09.006
Ng JH, Hirsch JS, Wanchoo R, Sachdeva M, Sakhiya V, Hong S, Jhaveri KD, Fishbane S, Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium (2020) Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int 98:1530–1539. https://doi.org/10.1016/j.kint.2020.07.030
Seidel M, Holzer B, Appel H, Babel N, Westhoff TH, COVID Dialysis Working Group (2020) Impact of renal disease and comorbidities on mortality in hemodialysis patients with COVID-19: a multicenter experience from Germany. J Nephrol 33:871–874. https://doi.org/10.1007/s40620-020-00828-8
Canpolat N, Yildirim ZY, Yildiz N, Tasdemir M, Goknar N, Evrengul H, Gulmez R, Aksu B, Dursun H, Ozcelik G, Yavascan O, Cicek RY, Tulpar S, Hacihamdioglu DO, Nayir A, Alpay H (2022) COVID-19 in pediatric patients undergoing chronic dialysis and kidney transplantation. Eur J Pediatr 181:117–123. https://doi.org/10.1007/s00431-021-04191-z
Saygili S, Canpolat N, Cicek RY, Agbas A, Yilmaz EK, Sakalli AAK, Aygun D, Akkoc G, Demirbas KC, Konukoglu D, Cokugras H, Caliskan S, Sever L (2022) Clinical and subclinical acute kidney injury in children with mild-to-moderate COVID-19. Pediatr Res. https://doi.org/10.1038/s41390-022-02124-6
Gasim GI, Bella A, Adam I (2015) Immune response to hepatitis B vaccine among patients on hemodialysis. World J Hepatol 7:270–275. https://doi.org/10.4254/wjh.v7.i2.270
Nailescu C, Xu X, Zhou H, Hall H, Wilson AC, Leiser JD, Chand DH, Valentini RP, Hebert D, Mahan JD (2011) Influenza vaccine after pediatric kidney transplant: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 26:459–467. https://doi.org/10.1007/s00467-010-1729-1
Reddy S, Chitturi C, Yee J (2019) Vaccination in chronic kidney disease. Adv Chronic Kidney Dis 26:72–78. https://doi.org/10.1053/j.ackd.2018.10.002
Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, Martzloff J, Perrin P, Moulin B, Fafi-Kremer S, Caillard S (2021) Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 99:1498–1500. https://doi.org/10.1016/j.kint.2021.04.005
Marinaki S, Adamopoulos S, Degiannis D, Roussos S, Pavlopoulou ID, Hatzakis A, Boletis IN (2021) Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant 21:2913–2915. https://doi.org/10.1111/ajt.16607
Rozen-Zvi B, Yahav D, Agur T, Zingerman B, Ben-Zvi H, Atamna A, Tau N, Mashraki T, Nesher E, Rahamimov R (2021) Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect 27:1173 e1171-1173 e1174. https://doi.org/10.1016/j.cmi.2021.04.028
Stumpf J, Siepmann T, Lindner T, Karger C, Schwobel J, Anders L, Faulhaber-Walter R, Schewe J, Martin H, Schirutschke H, Barnett K, Huther J, Muller P, Langer T, Pluntke T, Anding-Rost K, Meistring F, Stehr T, Pietzonka A, Escher K, Cerny S, Rothe H, Pistrosch F, Seidel H, Paliege A, Beige J, Bast I, Steglich A, Gembardt F, Kessel F, Kroger H, Arndt P, Sradnick J, Frank K, Klimova A, Mauer R, Grahlert X, Anft M, Blazquez-Navarro A, Westhoff TH, Stervbo U, Tonn T, Babel N, Hugo C (2021) Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur 9:100178. https://doi.org/10.1016/j.lanepe.2021.100178
Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T (2013) Serologic vaccination response after solid organ transplantation: a systematic review. PLoS ONE 8:e56974. https://doi.org/10.1371/journal.pone.0056974
Crespo M, Barrilado-Jackson A, Padilla E, Eguia J, Echeverria-Esnal D, Cao H, Faura A, Folgueiras M, Sola-Porta E, Pascual S, Barbosa F, Hurtado S, Ribera L, Rio-No L, Perez-Saez MJ, Redondo-Pachon D, Pascual J (2022) Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. Am J Transplant 22:786–800. https://doi.org/10.1111/ajt.16854
Sanders JF, Bemelman FJ, Messchendorp AL, Baan CC, van Baarle D, van Binnendijk R, Diavatopoulos DA, Frolke SC, Geers D, GeurtsvanKessel CH, den Hartog G, van der Heiden M, Imhof C, Kho MML, Koopmans MPG, Malahe SRK, Mattheussens WB, van der Molen R, van Mourik D, Remmerswaal EBM, Rots N, Vart P, de Vries RD, Gansevoort RT, Hilbrands LB, Reinders MEJ, RECOVAC Collaborators (2022) The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation 106:821–834. https://doi.org/10.1097/TP.0000000000003983
Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM (2021) Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 325:2204–2206. https://doi.org/10.1001/jama.2021.7489
Haskin O, Ashkenazi-Hoffnung L, Ziv N, Borovitz Y, Dagan A, Levi S, Koren G, Hamdani G, Levi-Erez D, Landau D, Alfandary H (2021) Serological response to the BNT162b2 COVID-19 mRNA vaccine in adolescent and young adult kidney transplant recipients. Transplantation 105:e226–e233. https://doi.org/10.1097/TP.0000000000003922
Crane C, Phebus E, Ingulli E (2022) Immunologic response of mRNA SARS-CoV-2 vaccination in adolescent kidney transplant recipients. Pediatr Nephrol 37:449–453. https://doi.org/10.1007/s00467-021-05256-9
Swai J, Gui M, Long M, Wei Z, Hu Z, Liu S (2022) Humoral and cellular immune response to severe acute respiratory syndrome coronavirus-2 vaccination in haemodialysis and kidney transplant patients. Nephrology (Carlton) 27:7–24. https://doi.org/10.1111/nep.13974
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP (2021) Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27:1205–1211. https://doi.org/10.1038/s41591-021-01377-8
Pedersen RM, Bang LL, Tornby DS, Kierkegaard H, Nilsson AC, Johansen IS, Bistrup C, Jensen TG, Justesen US, Andersen TE (2021) The SARS-CoV-2-neutralizing capacity of kidney transplant recipients 4 weeks after receiving a second dose of the BNT162b2 vaccine. Kidney Int 100:1129–1131. https://doi.org/10.1016/j.kint.2021.09.006
Bertrand D, Hamzaoui M, Lemee V, Lamulle J, Laurent C, Etienne I, Lemoine M, Lebourg L, Hanoy M, Le Roy F, Nezam D, Farce F, Plantier JC, Boyer O, Guerrot D, Candon S (2021) Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int 100:1337–1340. https://doi.org/10.1016/j.kint.2021.09.014
Boedecker-Lips SC, Lautem A, Runkel S, Klimpke P, Kraus D, Keil P, Holtz S, Tomalla V, Marczynski P, Boedecker CB, Galle PR, Koch M, Weinmann-Menke J (2022) Six-month follow-up after vaccination with BNT162b2: SARS-CoV-2 antigen-specific cellular and humoral immune responses in hemodialysis patients and kidney transplant recipients. Pathogens 11:67. https://doi.org/10.3390/pathogens11010067
Magicova M, Zahradka I, Fialova M, Neskudla T, Gurka J, Modos I, Hojny M, Raska P, Smejkal P, Striz I, Viklicky O (2022) Determinants of immune response to anti-SARS-CoV-2 mRNA vaccines in kidney transplant recipients: a prospective cohort study. Transplantation 106:842–852. https://doi.org/10.1097/TP.0000000000004044
Stumpf J, Tonnus W, Paliege A, Rettig R, Steglich A, Gembardt F, Kessel F, Kroger H, Arndt P, Sradnick J, Frank K, Tonn T, Hugo C (2021) Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation 105:e267–e269. https://doi.org/10.1097/TP.0000000000003903
Acknowledgements
We are thankful to Dr Omer Deniz Ocakli for assistance with data entry.
Funding
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University-Cerrahpasa (35866).
Author information
Authors and Affiliations
Contributions
Conceptualization: Salim Caliskan; methodology: Salim Caliskan, Bekir Kocazeybek, Ayca Kıykım; data collection: Ruveyda Gulmez, Bagdagul Aksu, Nurdan Yıldız, Diana Uckardes, Seha Saygılı, Esra Karabag Yılmaz, Zeynep Yuruk Yıldırım, Mehmet Tasdemir, Ahmet Nayır; material preparation and analysis were performed by Ruveyda Gulmez, Dogukan Ozbey; statistical analysis: Ayse Agbas; writing—original draft preparation: Ruveyda Gulmez, Ayse Agbas; writing—review and editing: Nur Canpolat, Salim Caliskan; supervision: Salim Caliskan, Bekir Kocazeybek, Haluk Cokugras. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Istanbul University-Cerrahpasa, Cerrahpasa School of Medicine (2021–70493).
Consent
Written informed consent was obtained from the parents and participants where available.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gulmez, R., Ozbey, D., Agbas, A. et al. Humoral and cellular immune response to SARS-CoV-2 mRNA BNT162b2 vaccine in pediatric kidney transplant recipients compared with dialysis patients and healthy children. Pediatr Nephrol 38, 2199–2208 (2023). https://doi.org/10.1007/s00467-022-05813-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05813-w