Abstract
Background
The underlying mechanisms of obesity in X-linked hypophosphatemia (XLH) are not known. We aimed to evaluate whether FGF21, an endocrine FGF involved in the regulation of carbohydrate–lipid metabolism, could be involved.
Methods
We performed a prospective multicenter cross-sectional study comparing FGF23, Klotho, and FGF21 levels in teenagers with XLH compared to healthy controls (VITADOS cohort) after matching for age, gender, and puberty. Non-parametric tests were performed (results presented as median (min–max)).
Results
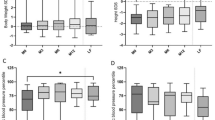
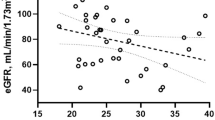
A total of 40 XLH teenagers (n = 20 Standard Of Care, SOC, n = 20 burosumab) were included. While patients receiving burosumab displayed increased BMI as compared to patients receiving SOC, systolic blood pressure expressed as percentile was progressively and significantly lower when comparing the three groups: 77 (4–99) in SOC, 47 (9–98) in burosumab, and 28 (1–94) in controls (p = 0.007). When compared to patients receiving SOC, patients receiving burosumab displayed significantly increased phosphate and 1,25(OH)2D levels. We found increased Klotho levels in patients receiving burosumab. No differences were found for either carbohydrate–lipid biomarkers or FGF21 between the three groups. A total of 21 XLH patients (53%) had insulin resistance (HOMA > 2.4, N = 10 SOC, N = 11 burosumab).
Conclusion
FGF21 does not explain obesity/overweight in XLH. Of note, this study was performed in France in 2018–2019, early after the approval authorizing burosumab only in case of severe XLH despite SOC. As such, the data on systolic blood pressure highlighting a possible impact of burosumab to decrease blood pressure as well as increase Klotho levels deserve further studies given their potential effect on long-term cardiovascular risk.
Graphical Abstract
A higher resolution version of the Graphical abstract is available as Supplementary information


Similar content being viewed by others
Data availability
Datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Haffner D, Emma F, Eastwood DM, Biosse Duplan M et al (2019) Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 15:435–455. https://doi.org/10.1038/s41581-019-0152-5
Bacchetta J, Bardet C, Prié D (2019) Physiology of FGF23 and overview of genetic diseases associated with renal phosphate wasting. Metabolism. https://doi.org/10.1016/j.metabol.2019.01.006
Rothenbuhler A, Fadel N, Debza Y, Bacchetta J et al (2019) High incidence of cranial synostosis and Chiari I malformation in children with X-linked hypophosphatemic rickets (XLHR). J Bone Miner Res 34:490–496. https://doi.org/10.1002/jbmr.3614
Kato H, Koga M, Kinoshita Y, Taniguchi Y et al (2021) Incidence of complications in 25 adult patients with X-linked hypophosphatemia. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgab282
Che H, Roux C, Etcheto A, Rothenbuhler A et al (2016) Impaired quality of life in adults with X-linked hypophosphatemia and skeletal symptoms. Eur J Endocrinol 174:325–333. https://doi.org/10.1530/EJE-15-0661
Zhukouskaya VV, Rothenbuhler A, Colao A, Di Somma C et al (2020) Increased prevalence of overweight and obesity in children with X-linked hypophosphatemia. Endocr Connect 9:144–153. https://doi.org/10.1530/EC-19-0481
Xie W, Méchin M-C, Dubois SG, Lajeunesse D et al (2002) Up-regulation of liver glucose-6-phosphatase in x-linked hypophosphatemic mice. Horm Metab Res Horm Stoffwechselforschung Horm Metab 34:288–292. https://doi.org/10.1055/s-2002-33256
Kharitonenkov A, DiMarchi R (2017) Fibroblast growth factor 21 night watch: advances and uncertainties in the field. J Intern Med 281:233–246. https://doi.org/10.1111/joim.12580
Badman MK, Koester A, Flier JS, Kharitonenkov A et al (2009) Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150:4931–4940. https://doi.org/10.1210/en.2009-0532
Henriksson E, Andersen B (2020) FGF19 and FGF21 for the treatment of NASH-two sides of the same coin? Differential and overlapping effects of FGF19 and FGF21 from mice to human. Front Endocrinol 11:601349. https://doi.org/10.3389/fendo.2020.601349
Carpenter TO, Insogna KL, Zhang JH, Ellis B et al (2010) Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab 95:E352-357. https://doi.org/10.1210/jc.2010-0589
Bacchetta J, Ginhoux T, Bernoux D, Dubourg L et al (2019) Assessment of mineral and bone biomarkers highlights a high frequency of hypercalciuria in asymptomatic healthy teenagers. Acta Paediatr 108:2253–2260. https://doi.org/10.1111/apa.14907
https://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html
Carpenter TO, Whyte MP, Imel EA, Boot AM et al (2018) Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med 378:1987–1998. https://doi.org/10.1056/NEJMoa1714641
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843. https://doi.org/10.2215/CJN.01640309
Ardeshirpour L, Cole DEC, Carpenter TO (2007) Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev 5(Suppl 1):584–598
Quinn SM, Baur LA, Garnett SP, Cowell CT (2010) Treatment of clinical insulin resistance in children: a systematic review. Obes Rev 11:722–730. https://doi.org/10.1111/j.1467-789X.2009.00697.x
Piketty M-L, Brabant S, Souberbielle J-C, Maruani G et al (2020) FGF23 measurement in burosumab-treated patients: an emerging treatment may induce a new analytical interference. Clin Chem Lab Med 58:e267–e269. https://doi.org/10.1515/cclm-2020-0460
Imel EA, Zhang X, Ruppe MD, Weber TJ et al (2015) Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab 100:2565–2573. https://doi.org/10.1210/jc.2015-1551
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51. https://doi.org/10.1038/36285
Cheikhi A, Barchowsky A, Sahu A, Shinde SN et al (2019) Klotho: an elephant in aging research. J Gerontol A Biol Sci Med Sci 74:1031–1042. https://doi.org/10.1093/gerona/glz061
Saar-Kovrov V, Donners MMPC, van der Vorst EPC (2020) Shedding of klotho: functional implications in chronic kidney disease and associated vascular disease. Front Cardiovasc Med 7:617842. https://doi.org/10.3389/fcvm.2020.617842
Forster RE, Jurutka PW, Hsieh J-C, Haussler CA et al (2011) Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun 414:557–562. https://doi.org/10.1016/j.bbrc.2011.09.117
Marcovecchio ML, Colombo M, Dalton RN, McKeigue PM et al (2020) Biomarkers associated with early stages of kidney disease in adolescents with type 1 diabetes. Pediatr Diabetes 21:1322–1332. https://doi.org/10.1111/pedi.13095
Faul C, Amaral AP, Oskouei B, Hu MC et al (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121:4393–4408. https://doi.org/10.1172/JCI46122
Andrukhova O, Slavic S, Smorodchenko A, Zeitz U et al (2014) FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6:744–759. https://doi.org/10.1002/emmm.201303716
Bacchetta J, Pelletier S (2018) Vitamin D deficiency is associated with mortality in maintenance dialysis: moving forward from epidemiology to clinical trials. Nephrol Dial Transplant 33:1679–1682
Bacchetta J, Sea JL, Chun RF, Lisse TS et al (2013) Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res 28:46–55. https://doi.org/10.1002/jbmr.1740
Chonchol M, Greene T, Zhang Y, Hoofnagle AN et al (2016) Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 27:227–237. https://doi.org/10.1681/ASN.2014101009
Hensel N, Schön A, Konen T, Lübben V et al (2016) Fibroblast growth factor 23 signaling in hippocampal cells: impact on neuronal morphology and synaptic density. J Neurochem 137:756–769. https://doi.org/10.1111/jnc.13585
Singh S, Grabner A, Yanucil C, Schramm K et al (2016) Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90:985–996. https://doi.org/10.1016/j.kint.2016.05.019
Alon US, Monzavi R, Lilien M, Rasoulpour M et al (2003) Hypertension in hypophosphatemic rickets–role of secondary hyperparathyroidism. Pediatr Nephrol 18:155–158. https://doi.org/10.1007/s00467-002-1044-6
Nakamura Y, Takagi M, Takeda R, Miyai K et al (2017) Hypertension is a characteristic complication of X-linked hypophosphatemia. Endocr J 64:283–289. https://doi.org/10.1507/endocrj.EJ16-0199
Nehgme R, Fahey JT, Smith C, Carpenter TO (1997) Cardiovascular abnormalities in patients with X-linked hypophosphatemia. J Clin Endocrinol Metab 82:2450–2454. https://doi.org/10.1210/jcem.82.8.4181
Hernández-Frías O, Gil-Peña H, Pérez-Roldán JM, Gonzalez-Sanchez S et al (2019) Risk of cardiovascular involvement in pediatric patients with X-linked hypophosphatemia. Pediatr Nephrol 34:1077–1086. https://doi.org/10.1007/s00467-018-4180-3
Takashi Y, Kinoshita Y, Hori M, Ito N et al (2017) Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr Res 42:132–137. https://doi.org/10.1080/07435800.2016.1242604
Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A et al (2016) 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34:1887–1920. https://doi.org/10.1097/HJH.0000000000001039
Acknowledgements
The authors would like to acknowledge the clinical research associates from the different Reference Centers for their help to conduct this study.
Funding
The Hospices Civils de Lyon received a research grant from Kyowa Kirin for the XLH21 study. An institutional funding for the VITADOS cohort was provided by the Programme Hospitalier de Recherche Clinique Inter-régional (PHRCi 2011, JB). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JB and JPS received travel fees and consultant/speaker fees from Kyowa Kirin. JB is an investigator for clinical trials for Kyowa Kirin. AL is an investigator for clinical trials for Kyowa Kirin and her research team receives research grants from Kyowa Kirin.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Graphical abstract
(PPTX 52 KB)
Supplementary file 2
(DOCX 295 KB)
Rights and permissions
About this article
Cite this article
Bloudeau, L., Linglart, A., Flammier, S. et al. X-linked hypophosphatemia, obesity and arterial hypertension: data from the XLH21 study. Pediatr Nephrol 38, 697–704 (2023). https://doi.org/10.1007/s00467-022-05636-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05636-9