Abstract
Background
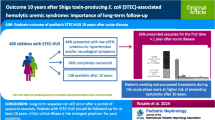
Hemoconcentration has been identified as a risk factor for a complicated course in Shiga toxin-producing E. coli-hemolytic uremic syndrome (STEC-HUS). This single-center study assesses hemoconcentration and predictors at presentation in STEC-HUS treated from 2009–2017.
Methods
Data of 107 pediatric patients with STEC-HUS were analyzed retrospectively. Patients with mild HUS (mHUS, definition: max. serum creatinine < 1.5 mg/dL and no major neurological symptoms) were compared to patients with severe HUS (sHUS, definition: max. serum creatinine ≥ 1.5 mg/dL ± major neurological symptoms). Additionally, predictors of complicated HUS (dialysis ± major neurological symptoms) were analyzed.
Results
Sixteen of one hundred seven (15%) patients had mHUS. Admission of patients with sHUS occurred median 2 days earlier after the onset of symptoms than in patients with mHUS. On admission, patients with subsequent sHUS had significantly higher median hemoglobin (9.5 g/dL (3.6–15.7) vs. 8.5 g/dL (4.2–11.5), p = 0.016) than patients with mHUS. The product of hemoglobin (g/dL) and LDH (U/L) (cutoff value 13,302, sensitivity 78.0%, specificity of 87.5%) was a predictor of severe vs. mild HUS. Creatinine (AUC 0.86, 95% CI 0.79–0.93) and the previously published score hemoglobin (g/dL) + 2 × creatinine (mg/dL) showed a good prediction for development of complicated HUS (AUC 0.87, 95% CI 0.80–0.93).
Conclusions
At presentation, patients with subsequent severe STEC-HUS had a higher degree of hemoconcentration. This underlines that fluid loss or reduced fluid intake/administration may be a risk factor for severe HUS. The good predictive value of the score hemoglobin (g/dL) + 2 × creatinine (mg/dL) for complicated HUS could be validated in our cohort.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary Information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal infections with Shiga toxin-producing Escherichia coli (STEC) can lead to (hemorrhagic) colitis and, usually after several days of gastrointestinal prodomi, be complicated by hemolytic uremic syndrome (HUS) [1]. Shiga toxin (Stx) plays a key role in the pathogenesis of STEC-HUS. After ingestion and intestinal infection, Stx is translocated from the gut into the bloodstream. Once in the bloodstream, Stx induces systemic proinflammatory and prothrombotic pathways [2]. For the broad spectrum of other causes of HUS, we refer to recent review articles [3, 4].
STEC-HUS mainly affects children below the age of 5 years and is defined by non-immune hemolytic anemia, thrombocytopenia, and acute kidney injury (AKI) in the context of STEC infection [1]. Due to the preceding several days of gastroenteritis, most patients are dehydrated and hemoconcentrated at the onset of STEC-HUS. Thus, beside the direct effects of Stx, a prerenal component might contribute to AKI [5]. STEC-HUS can affect multiple organs, e.g., the kidney and the brain. This can result in severe neurological complications. Therapy of STEC-HUS is mainly supportive including fluid management, transfusions, treatment of hypertension, kidney replacement therapy (KRT), and treatment of neurological complications. Most patients recover completely. However, the case fatality rate of STEC-HUS is around 1–5%. Additionally, follow-up data from Germany and Austria show relevant sequelae of STEC-HUS with chronic kidney disease (CKD) ≥ stage 2 in 4–7% and potentially major neurological sequelae [6, 7]. Thus, the management of STEC-HUS has to be further optimized.
Several easily accessible clinical parameters have been evaluated or identified as prognostic markers in STEC-HUS. This includes parameters of thromboinflammation (e.g., leukocytosis [8,9,10], elevated D-dimers [11], complement activation [12]), urea [12], sodium [13], and lactate dehydrogenase (LDH) [14,15,16].
Additionally, high hematocrit or hemoglobin levels as surrogate parameters of hemoconcentration have been associated with developing STEC-HUS or a more severe course of STEC-HUS [5, 9, 13, 15, 17,18,19,20]. Based on these findings, Ardissino et al. suggested a score (hemoglobin (g/dL) + 2 × creatinine (mg/dL) on admission) to identify patients at high risk for a severe course. The score was moderately predictive for a complicated course of HUS (AUC 0.75, 95% CI 0.67–0.85), defined by the outcome death or CKD stage 2–5 [18]. Recently, Lin et al. validated the score for the end-points dialysis, neurologic complications, respiratory failure, and death (AUC 0.77, 95% CI 0.68–0.87) [21].
Several studies have shown that volume expansion with infusion therapy improves the outcome. Early initiation of volume expansion during the gastrointestinal phase reduces the risk to develop HUS at all [15] or oligoanuric HUS [5, 22, 23]. Volume expansion in established STEC-HUS mitigates the course of the disease, eventually reducing the need for dialysis and the rate of neurological complications. In a cohort of 38 patients treated by infusion therapy and compared to historical controls, Ardissino et al. showed that neurological involvement (8% vs. 24%, p = 0.06) and KRT (26% vs. 58%, p = 0.01) could be reduced [24]. Recently, Bonany et al. published a similar study showing a reduced need for dialysis in patients treated with fluid infusion (n = 16, dialysis in 12.5%) compared to historical controls treated by fluid restriction (n = 19, dialysis in 47%) [25].
Due to the fact that some patients are severely dehydrated and hemoconcentrated at the beginning of STEC-HUS with a subsequent apparent normal hemoglobin level, it has also been questioned whether the classical definition of STEC-HUS should be applied at all stages of the disease and if this definition might reduce the chance to identify patients with STEC-HUS early [15, 26]. Additionally, not all patients do fulfill all three abovementioned classical criteria for HUS at onset or even during the course of HUS [26, 27]. Especially in milder courses of HUS, the AKI might be accompanied by only a slight increase of creatinine.
Based on the hypothesis that a severe course of the disease is associated with more hemoconcentration, the aim of this study was to evaluate the initial hemoconcentration and predictors for the course of HUS in our single-center cohort.
Methods
Patients
Medical records of all patients treated for STEC-HUS at our center between 2009 and 2017 were analyzed retrospectively. Patients with atypical/complement-, pneumococcal-, and pertussis-associated HUS (n = 8) were excluded. Some data of 33 patients included in this study have been published previously [28].
Microbiologic analysis for STEC infection was based on the local protocol including (enriched) stool cultures for STEC and immunoassay or polymerase chain reaction (PCR) for detection of Stx.
The following clinical definitions were applied:
-
HUS: hemolytic anemia (minimum hemoglobin below the lower limit of the normal range), thrombocytopenia (minimum thrombocytes < 150 × 109/L), and serum creatinine above the upper limit of the age-dependent normal range. Two patients had minimum thrombocytes > 150 × 109/L but showed platelet consumption.
-
Severe HUS (sHUS): HUS with a maximum serum creatinine ≥ 1.5 mg/dL ± major neurological symptoms (definition: impairment of consciousness (stupor or coma), epileptic seizures, focal neurological deficits (e.g., paresis), and/or visual disturbances (double or blurry vision)) during the course of the disease.
-
Mild HUS (mHUS): HUS with a maximum serum creatinine < 1.5 mg/dL (reflecting the KDIGO-criteria for the AKI stage 1 [29]) and absence of major neurological symptoms.
The local ethical committee approved the study (PV3975, WF-016/19).
Statistics
Descriptive statistics are presented for continuous variables (median and range) and for categorical variables (number and percentage). Continuous variables were compared using the Mann–Whitney U test for two groups or the Kruskal–Wallis test followed by the Dunn procedure for multiple groups. A receiver operating characteristic (ROC) curve was plotted for specific laboratory parameters. Pearson’s χ2 test was used for categorical data. P values < 0.05 were considered statistically significant. Data were analyzed using PRISM (Version 8, GraphPad, USA).
Results
Cohort
A cohort of 107 patients with STEC-HUS was analyzed (females: 58 (54%), males: 49 (46%)). The median age was 4.0 years (0.5–16.6). STEC or Stx in the stool could be detected in 79 (74%) patients by culture and/or PCR/immunoassay.
Major neurological symptoms occurred in 35 (33%) patients. Mortality was 3/107 (3%) due to neurological complications (cerebral bleeding, edema).
Mild HUS vs. severe HUS
Patients were divided into two groups according to the definitions given above.
Sixteen (15%) patients had mild HUS (mHUS). In all patients, maximum serum creatinine was < 1.5 mg/dL. In 11/16 patients, it was even < 1.0 mg/dL. None received dialysis.
Ninety-one (85%) patients had severe HUS (sHUS). In total, KRT was performed in 61 (57%) patients (61/91 (67%) in the sHUS group). The median duration of dialysis was 10 days in those where dialysis could be terminated. Three patients were on dialysis at discharge. Three patients in the sHUS group had a max. creatinine < 1.5 mg/dL but major neurological symptoms.
Initial hemoconcentration in sHUS vs. mHUS
Patients with mHUS were admitted later after onset of symptoms (diarrhea, vomiting, abdominal pain, and/or fever) than patients with sHUS (Table 1). Compared to mHUS cases, patients with sHUS had a significantly higher hemoglobin (Table 1) and hematocrit (27.3% (10.0–44.2) vs. 23.6% (12.4–33.5), p = 0.016) on admission. This was even significant after adjustment by calculation of absolute and relative (percentage) hemoglobin reduction from the lower limit of the age-dependent reference values (data not shown).
When the sHUS group was divided into patients with and without dialysis and those groups were compared to mHUS patients, there was a significant difference in the hemoglobin level at admission between mHUS and sHUS with dialysis groups (Fig. 1a). Thus, the highest hemoglobin levels on admission were associated with the most severe course of disease regarding kidney involvement.
At presentation and during the course of the disease, a higher LDH in the sHUS group compared to mHUS was observed (Table 1). However, the hemoglobin nadir and the hemoglobin at discharge did not significantly differ between patients with mHUS and sHUS (Table 1).
Ninety of one hundred seven patients (84%) received red blood cell transfusions. The proportion of patients receiving red blood cell transfusions was comparable between mHUS and sHUS patients (14/16 vs. 76/91 (OR 0.72 (95% CI 0.15–2.94), p = 0.69)). The total median number of red blood cell transfusions per patient was only slightly higher in the sHUS group (1 (0–2) vs. 1 (0–18), p = 0.042).
Predictors of mild vs. severe HUS
Hemoglobin or LDH on admission alone was moderately predictive for sHUS (AUC 0.69 and AUC 0.76, respectively, data not shown). However, the product of hemoglobin and LDH (Hb (g/dL) × LDH (U/L)) on admission was able to distinguish subsequent sHUS from mHUS (AUC 0.82, 95% CI 0.69–0.94). A cutoff value of 13,302 had a sensitivity of 78.0% (95% CI 68.5–85.3%) and a specificity of 87.5% (95% CI 64.0–97.8%) (Fig. 2a).
ROC curves the product of hemoglobin and lactate dehydrogenase (Hb (g/dL) × LDH (U/L)) on admission to predict development of severe vs. mild HUS (a) as well as ROC curves for creatinine (mg/dL) on admission (b) and hemoglobin (g/dL) + 2 × creatinine (mg/dL) on admission (c) to predict complicated HUS
Predictors of complicated HUS
Patients with major neurological symptoms showed a significantly higher hemoglobin on admission than patients without neurological complications (Fig. 1b).
Patients with a complicated course of HUS (dialysis and/or major neurological symptoms) (n = 66) did not show a significantly higher hemoglobin on admission than patients without these complications (n = 41) (9.5 g/dL (4.8–13.8) vs. 8.8 g/dL (3.6–15.7), p = 0.06). However, serum creatinine (4.3 mg/dL (0.3–18.0) vs. 1.3 mg/dL (0.3–3.9), p < 0.0001) and LDH (2513 U/L (301–5397) vs. 1574 U/L (258–3159), p < 0.0001) on admission were significantly higher in patients with subsequently complicated HUS.
Therefore, serum creatinine (mg/dL) on admission was predictive for complicated HUS (AUC 0.86, 95% CI 0.79–0.93, Fig. 2b). Hb (g/dL) × LDH (U/L) on admission was moderately predictive (AUC 0.80, 95% CI 0.72–0.89, cutoff values not shown).
The previous established score hemoglobin (g/dL) + 2 × creatinine (mg/dL) on admission [18] showed a good predictive value for development of a complicated HUS (AUC 0.87, 95% CI 0.80–0.93, Fig. 2c). A cutoff value of 14.2 had a sensitivity of 80.3% (95% CI 69.2–88.1%) and a specificity of 73.2% (95% CI 58.1–84.3%).
Discussion
This single-center, long-term study shows that patients with sHUS had higher hemoglobin and hematocrit levels on admission than patients with mHUS, indicating that patients with sHUS had profound hemoconcentration.
Furthermore, the highest values on admission occurred in patients with subsequent need for dialysis or with major neurological symptoms. Thus, hemoconcentration on admission is associated with disease activity and prognosis in STEC-HUS. However, we did not find a difference between groups for the combined end-point dialysis and/or major neurological complications (complicated HUS).
These findings could be confounded by the time point of admission since patients with mHUS were admitted later than patients with sHUS and the drop of the hemoglobin level could be more advanced due to ongoing hemolysis. In this case, a higher LDH might be expected. In contrast, LDH, which reflects hemolysis, was higher in sHUS patients vs. mHUS patients on admission. This should contribute to lowering the hemoglobin value in sHUS patients. However, LDH could also be increased due to multi-organ involvement in sHUS patients. The association of LDH with the more severe disease has been reported previously [14,15,16]. In our cohort, the product of hemoglobin and LDH on admission was a predictor of sHUS.
Interestingly, a later admission in milder cases of HUS was recently also observed by McKee et al. [15]. Alconcher et al. also reported an earlier admission in patients who died of STEC-HUS compared to survivors [13].
The score applied by Ardissino et al. (hemoglobin (g/dL) + 2 × creatinine (mg/dL)) was moderately predictive for a complicated course of HUS in their cohort. However, clinical definitions vary substantially between the study by Ardissino et al. and our definition of sHUS since in this series the complicated course of HUS was defined by death and the long-term kidney outcome (CKD stage 2–5) [18].
To validate the score established by Ardissino et al., we analyzed our cohort for its predictive value for complicated HUS defined by dialysis and/or major neurological symptoms. We found a good prediction with an AUC of 0.87. This was even better than in the recent study published by Lin et al. in 155 patients, in which they calculated a AUC of 0.71 using similar end-points to our study [21].
Incomplete or mild forms of STEC-HUS have been reported in up to 65% of the patients and no AKI was present upon admission in 14% of the patients [16, 26]. This has led to the discussion of the classical HUS criteria. However, data on the prevalence of mild forms of STEC-HUS in larger cohorts based on a clear case definition are lacking. To evaluate the prevalence of mHUS in our cohort, we defined kidney involvement in mHUS cases by a creatinine cutoff of < 1.5 mg/dL. This is equal to twice the maximum upper limit of serum creatinine across all age groups in our cohort and, since pre-HUS creatinine values are lacking in our patients, reflects the definition of AKI stage 1 according to the KDOQI guidelines most accurately [29]. In our cohort, a substantial number of patients (15%) had a mild form of HUS with only minimal kidney involvement and no major neurological symptoms during the course of the disease.
Interestingly, the minimum hemoglobin and the rate of transfusions did not differ between mild and severe HUS. However, the minimum hemoglobin could be confounded by the indication for transfusions. The number of transfusions per patient was slightly higher in the group with sHUS. In contrast, Cobenas et al. found a more pronounced association for the need for red blood cell transfusions with more severe kidney disease [14].
It remains unclear why some patients with STEC infection do not develop HUS at all and some are less prone to a more severe course of HUS. Unfortunately, the cause of the lower hemoconcentration in patients with mHUS could not be identified in our retrospective study. Probably, these patients were less affected in the gastrointestinal prodromal phase and had less fluid loss and/or a better oral fluid intake before admission. Even though our study was retrospective and interventional data is lacking, our data indirectly support the concept of infusion therapy/volume expansion in patients with STEC infection or HUS to prevent or treat intravascular volume depletion and improve organ perfusion and ultimately improve the course and outcome of STEC-HUS [5].
In conclusion, patients with subsequent sHUS have a higher degree of hemoconcentration on admission and sHUS can be predicted by Hb × LDH on admission. We could validate the predictive value of the score hemoglobin (g/dL) + 2 × creatinine (mg/dL) for complicated HUS. Since the correction of hemoconcentration seems to be a simple and effective therapeutic measure, further prospective studies combining treatment and rheological data are needed to strengthen this concept.
Abbreviations
- AKI:
-
acute kidney injury
- CKD:
-
chronic kidney disease
- HUS :
-
hemolytic uremic syndrome
- Hb:
-
hemoglobin
- LDH:
-
lactate dehydrogenase
- mHUS:
-
mild hemolytic uremic syndrome
- KRT:
-
kidney replacement therapy
- sHUS:
-
severe hemolytic uremic syndrome
- STEC :
-
Shiga toxin-producing Escherichia coli
- Stx :
-
Shiga toxin
References
Tarr PI, Gordon CA, Chandler WL (2005) Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086. https://doi.org/10.1016/S0140-6736(05)71144-2
Exeni RA, Fernandez-Brando RJ, Santiago AP, Fiorentino GA, Exeni AM, Ramos MV, Palermo MS (2018) Pathogenic role of inflammatory response during Shiga toxin-associated hemolytic uremic syndrome (HUS). Pediatr Nephrol 33:2057–2071. https://doi.org/10.1007/s00467-017-3876-0
Karpman D, Loos S, Tati R, Arvidsson I (2017) Haemolytic uraemic syndrome. J Intern Med 281:123–148. https://doi.org/10.1111/joim.12546
Loirat C, Saland J, Bitzan M (2012) Management of hemolytic uremic syndrome. Presse Med 41:e115–e135. https://doi.org/10.1016/j.lpm.2011.11.013
Grisaru S, Xie J, Samuel S, Hartling L, Tarr PI, Schnadower D, Freedman SB, Alberta Provincial Pediatric Enteric Infection Team (2017) Associations between hydration status, intravenous fluid administration, and outcomes of patients infected with Shiga toxin-producing Escherichia coli: a systematic review and meta-analysis. JAMA Pediatr 171:68–76. https://doi.org/10.1001/jamapediatrics.2016.2952
Rosales A, Hofer J, Zimmerhackl LB, Jungraithmayr TC, Riedl M, Giner T, Strasak A, Orth-Holler D, Wurzner R, Karch H (2012) Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin Infect Dis 54:1413–1421. https://doi.org/10.1093/cid/cis196
Loos S, Aulbert W, Hoppe B, Ahlenstiel-Grunow T, Kranz B, Wahl C, Staude H, Humberg A, Benz K, Krause M, Pohl M, Liebau MC, Schild R, Lemke J, Beringer O, Muller D, Hartel C, Wigger M, Vester U, Konrad M, Haffner D, Pape L, Oh J, Kemper MJ (2017) Intermediate follow-up of pediatric patients with hemolytic uremic syndrome during the 2011 outbreak caused by E. coli O104:H4. Clin Infect Dis 64:1637–1643. https://doi.org/10.1093/cid/cix218
Buteau C, Proulx F, Chaibou M, Raymond D, Clermont MJ, Mariscalco MM, Lebel MH, Seidman E (2000) Leukocytosis in children with Escherichia coli O157:H7 enteritis developing the hemolytic-uremic syndrome. Pediatr Infect Dis J 19:642–647
Mody RK, Gu W, Griffin PM, Jones TF, Rounds J, Shiferaw B, Tobin-D’Angelo M, Smith G, Spina N, Hurd S, Lathrop S, Palmer A, Boothe E, Luna-Gierke RE, Hoekstra RM (2015) Postdiarrheal hemolytic uremic syndrome in United States children: clinical spectrum and predictors of in-hospital death. J Pediatr 166:1022–1029. https://doi.org/10.1016/j.jpeds.2014.12.064
Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, Watkins SL, Tarr PI (2012) Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin Infect Dis 55:33–41. https://doi.org/10.1093/cid/cis299
Chandler WL, Jelacic S, Boster DR, Ciol MA, Williams GD, Watkins SL, Igarashi T, Tarr PI (2002) Prothrombotic coagulation abnormalities preceding the hemolytic-uremic syndrome. N Engl J Med 346:23–32. https://doi.org/10.1056/NEJMoa011033
Balestracci A, Meni Bataglia L, Toledo I, Beaudoin L, Alvarado C (2020) C3 levels and acute outcomes in Shiga toxin-related hemolytic uremic syndrome. Pediatr Nephrol 35:331–339. https://doi.org/10.1007/s00467-019-04334-3
Alconcher LF, Coccia PA, Suarez ADC, Monteverde ML, Perez YGMG, Carlopio PM, Missoni ML, Balestracci A, Principi I, Ramirez FB, Estrella P, Micelli S, Leroy DC, Quijada NE, Seminara C, Giordano MI, Hidalgo Solis SB, Saurit M, Caminitti A, Arias A, Rivas M, Risso P, Liern M (2018) Hyponatremia: a new predictor of mortality in patients with Shiga toxin-producing Escherichia coli hemolytic uremic syndrome. Pediatr Nephrol 33:1791–1798. https://doi.org/10.1007/s00467-018-3991-6
Cobenas CJ, Bresso PS, Lombardi LL, Amoreo OR, Ruscasso JD, Spizzirri AP, Del CSA, Zalba JH, Rahman RC, Risso P (2015) Relationship between red blood cell transfusion requirements and severity of renal disease during the acute stage of hemolytic uremic syndrome. Pediatr Nephrol 30:2115–2119. https://doi.org/10.1007/s00467-015-3147-x
McKee RS, Schnadower D, Tarr PI, Xie J, Finkelstein Y, Desai N, Lane RD, Bergmann KR, Kaplan RL, Hariharan S, Cruz AT, Cohen DM, Dixon A, Ramgopal S, Rominger A, Powell EC, Kilgar J, Michelson KA, Beer D, Bitzan M, Pruitt CM, Yen K, Meckler GD, Plint AC, Bradin S, Abramo TJ, Gouin S, Kam AJ, Schuh A, Balamuth F, Hunley TE, Kanegaye JT, Jones NE, Avva U, Porter R, Fein DM, Louie JP, Freedman SB, Pediatric Emergency Medicine Collaborative Research Committee and Pediatric Emergency Research Canada (2020) Predicting hemolytic uremic syndrome and renal replacement therapy in Shiga toxin-producing Escherichia coli-infected Children. Clin Infect Dis 70:1643–1651. https://doi.org/10.1093/cid/ciz432
Yamamoto T, Satomura K, Okada S, Ozono K (2009) Risk factors for neurological complications in complete hemolytic uremic syndrome caused by Escherichia coli O157. Pediatr Int 51:216–219. https://doi.org/10.1111/j.1442-200X.2008.02690.x
Ardissino G, Dacco V, Testa S, Civitillo CF, Tel F, Possenti I, Belingheri M, Castorina P, Bolsa-Ghiringhelli N, Tedeschi S, Paglialonga F, Salardi S, Consonni D, Zoia E, Salice P, Chidini G (2014) Hemoconcentration: a major risk factor for neurological involvement in hemolytic uremic syndrome. Pediatr Nephrol 30:345–352. https://doi.org/10.1007/s00467-014-2918-0
Ardissino G, Tel F, Testa S, Paglialonga F, Longhi S, Martelli L, Consolo S, Picicco D, Dodaro A, Daprai L, Colombo R, Arghittu M, Perrone M, Chidini G, Scalia Catenacci S, Cropanese I, Consonni D, ItalKid HUS Network (2018) A simple prognostic index for Shigatoxin-related hemolytic uremic syndrome at onset: data from the ItalKid-HUS network. Eur J Pediatr 177:1667–1674. https://doi.org/10.1007/s00431-018-3198-7
Balestracci A, Martin SM, Toledo I, Alvarado C, Wainsztein RE (2012) Dehydration at admission increased the need for dialysis in hemolytic uremic syndrome children. Pediatr Nephrol 27:1407–1410. https://doi.org/10.1007/s00467-012-2158-0
Oakes RS, Siegler RL, McReynolds MA, Pysher T, Pavia AT (2006) Predictors of fatality in postdiarrheal hemolytic uremic syndrome. Pediatrics 117:1656–1662. https://doi.org/10.1542/peds.2005-0785
Lin CY, Xie J, Freedman SB, McKee RS, Schnadower D, Tarr PI, Finkelstein Y, Desai NM, Lane RD, Bergmann KR, Kaplan RL, Hariharan S, Cruz AT, Cohen DM, Dixon A, Ramgopal S, Powell EC, Kilgar J, Michelson KA, Bitzan M, Yen K, Meckler GD, Plint AC, Balamuth F, Bradin S, Gouin S, Kam AJ, Meltzer JA, Hunley TE, Avva U, Porter R, Fein DM, Louie JP, Tarr GAM, Pediatric Emergency Research Canada (PERC) and Pediatric Emergency Medicine Collaborative Research Committee (PEMCRC) STEC Study Group (2021) Predicting adverse outcomes for Shiga toxin-producing Escherichia coli infections in emergency departments. J Pediatr. https://doi.org/10.1016/j.jpeds.2020.12.077
Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, Klein EJ, Tarr PI (2005) Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 115:e673–e680. https://doi.org/10.1542/peds.2004-2236
Hickey CA, Beattie TJ, Cowieson J, Miyashita Y, Strife CF, Frem JC, Peterson JM, Butani L, Jones DP, Havens PL, Patel HP, Wong CS, Andreoli SP, Rothbaum RJ, Beck AM, Tarr PI (2011) Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch Pediatr Adolesc Med 165:884–889. https://doi.org/10.1001/archpediatrics.2011.152
Ardissino G, Tel F, Possenti I, Testa S, Consonni D, Paglialonga F, Salardi S, Borsa-Ghiringhelli N, Salice P, Tedeschi S, Castorina P, Colombo RM, Arghittu M, Daprai L, Monzani A, Tozzoli R, Brigotti M, Torresani E (2016) Early volume expansion and outcomes of hemolytic uremic syndrome. Pediatrics 137:1–9. https://doi.org/10.1542/peds.2015-2153
Bonany P, Bilkis MD, Iglesias G, Braun A, Tello J, Ratto V, Vargas A, Koch E, Jannello P, Monteverde E (2021) Fluid restriction versus volume expansion in children with diarrhea-associated HUS: a retrospective observational study. Pediatr Nephrol 36:103–109. https://doi.org/10.1007/s00467-020-04673-6
Ardissino G, Possenti I, Tel F, Testa S, Paglialonga F (2014) Time to change the definition of hemolytic uremic syndrome. Eur J Intern Med 25:e29. https://doi.org/10.1016/j.ejim.2013.12.002
Yoshioka K, Yagi K, Moriguchi N (1999) Clinical features and treatment of children with hemolytic uremic syndrome caused by enterohemorrhagic Escherichia coli O157:H7 infection: experience of an outbreak in Sakai City, 1996. Pediatr Int 41:223–227
Loos S, Ahlenstiel T, Kranz B, Staude H, Pape L, Hartel C, Vester U, Buchtala L, Benz K, Hoppe B, Beringer O, Krause M, Muller D, Pohl M, Lemke J, Hillebrand G, Kreuzer M, Konig J, Wigger M, Konrad M, Haffner D, Oh J, Kemper MJ (2012) An outbreak of Shiga toxin-producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: presentation and short-term outcome in children. Clin Infect Dis 55:753–759. https://doi.org/10.1093/cid/cis531
KDIGO (2012) KDIGO Clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1. https://doi.org/10.1038/kisup.2012.1
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Sebastian Loos and Laura van de Loo. The first draft of the manuscript was written by Sebastian Loos and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The local ethical committee approved the study (PV3975, WF-016/19).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PPTX 114 kb).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loos, S., Oh, J., van de Loo, L. et al. Hemoconcentration and predictors in Shiga toxin-producing E. coli-hemolytic uremic syndrome (STEC-HUS). Pediatr Nephrol 36, 3777–3783 (2021). https://doi.org/10.1007/s00467-021-05108-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05108-6