Abstract
Background
Henoch–Schönlein purpura (HSP) nephritis and primary IgA nephropathy (pIgAN) present with glomerular IgA deposits, but differ with regard to clinical features. The suspected involvement of different immune system pathways is largely unknown.
Methods
This study was aimed at investigating some of the immunological features including Toll-like receptors (TLR), proteasome (PS)/immunoproteasome (iPS) switch, and the regulatory T cell system (Treg/Th17 cells) in 63 children with HSP with/without renal involvement and in 25 with pIgAN. Real-time PRC (Taqman) was used to quantify mRNA levels in peripheral blood mononuclear cells (PBMC).
Results
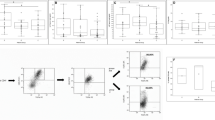
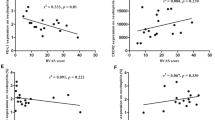
The expression of mRNAs encoding for TLR4 in both HSP and pIgAN was higher than in controls (HC) and in both diseases FoxP3mRNA and TGF-β1mRNA expression was significantly lower than in HC. A switch from PS to iPS (LMP2/β1) was detected only in PBMC of HSP and it correlated with the level of TLR2mRNA, which was selectively increased only in children with HSP.
Conclusion
Children with HSP and pIgAN present with similar signs of engagement of the innate immunity and regulatory T cell depression. The increased immunoproteasome switch, which correlated with TLR2 activation, may suggest an innate immunity pathway peculiar to HSP vasculitic presentation. This research area also deserves further investigation for possible therapeutic applications.



Similar content being viewed by others
References
Davin JC (2011) Henoch-Schonlein purpura nephritis: pathophysiology, treatment, and future strategy. Clin J Am Soc Nephrol 6:679–689
Coppo R (2008) Pediatric IgA nephropathy: clinical and therapeutic perspectives. Semin Nephrol 28:18–26
Davin JC, Ten Berge IJ, Weening JJ (2001) What is the difference between IgA nephropathy and Henoch-Schönlein purpura nephritis? Kidney Int 59:823–834
Calvo-Río V, Loricera J, Martín L, Ortiz-Sanjuán F, Alvarez L, González-Vela MC, González-Lamuño D, Mata C, Gortázar P, Rueda-Gotor J, Arias M, Peiró E, Martínez-Taboada VM, González-Gay MA, Blanco R (2013) Henoch-Schönlein purpura nephritis and IgA nephropathy: a comparative clinical study. Clin Exp Rheumatol 31:S45–S51
Bogdanovic R (2009) Henoch-Schönlein purpura nephritis in children: Risk factors, prevention and treatment. Acta Paediatr 98:1882–1889
Coppo R, Mazzucco G, Cagnoli L, Lupo A, Schena FP (1997) Longterm prognosis of Henoch-Schönlein nephritis in adults and children. Italian Group of Renal Immunopathology Collaborative Study on Henoch-Schönlein purpura. Nephrol Dial Transplant 12:2277–2283
Edström Halling S, Söderberg MP, Berg UB (2012) Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification). Nephrol Dial Transplant 27:715–722
Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Sako M, Kaito H, Nozu K, Tanaka R, Iijima K, Yoshikawa N (2013) Spontaneous remission in children with IgA nephropathy. Pediatr Nephrol 28:71–76
Novak J, Julian BA, Mestecky J, Renfrow MB (2012) Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol 34:365–382
Lau KK, Wyatt RJ, Moldoveanu Z, Tomana M, Julian BA, Hogg RJ, Lee JY, Huang WQ, Mestecky J, Novak J (2007) Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr Nephrol 22:2067–2072
Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chathan WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J (2009) Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119:1668–1677
Wyatt RJ, Julian BA (2013) IgA nephropathy. N Engl J Med 368:2402–2414
Coppo R, Amore A, Peruzzi L, Vergano L, Camilla R (2010) Innate immunity and IgA nephropathy. J Nephrol 23:626–632
Rigante D, Castellazzi L, Bosco A, Esposito S (2013) Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev 12:1016–1021
Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449:819–826
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Beutler BA (2009) TLRs and innate immunity. Blood 113:1399–1407
Rock KL, York IA, Saric T (2002) Protein degradation and generation of MHC class I-presented peptides. Adv Immunol 80:1–70
Macagno A, Kuehn L, de Giuli R, Groettrup M (2001) Pronounced up-regulation of the PA28alpha/β proteasome regulator but little increase in the steady state. Content of immunoproteasome during dendritic cell maturation. Eur J Immunol 31:3271–3280
Pedruzzi LM, Stockler-Pinto MB, Leite M Jr, Mafra D (2012) Nrf2-keap1 system versus NF-κB: the good and the evil in chronic kidney disease? Biochimie 94:2461–2466
Sakaguchi S, Wing K, Miyara M (2007) Regulatory T cells—a brief history and perspective. Eur J Immunol 37 [Suppl 1]:S116–S123
Beyer M, Schultze JL (2011) Plasticity of Treg cells: is reprogramming of Treg cells possible in the presence of FOXP3? Int Immunopharmacol 11:555–560
Turner JE, Paust HJ, Steinmetz OM, Panzer U (2010) The Th17 immune response in renal inflammation. Kidney Int 77:1070–1075
Coarthay A (2009) How do regulatory T cells work? Scand J Immunol 70:326–366
Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, Buoncompagni A, Lazar C, Bilge I, Uziel Y, Rigante D, Cantarini L, Hilario MO, Silva CA, Alegria M, Norambuena X, Belot A, Berkun Y, Estrella AI, Olivieri AN, Alpigiani MG, Rumba I, Sztajnbok F, Tambic-Bukovac L, Breda L, Al-Mayouf S, Mihaylova D, Chsnyk V, Sengler C, Klein-Gitelman M, Djeddi D, Nuno L, Pruunsild C, Brunner J, Kondi A, Pagava K, Pederzoli S, Ruperto N (2010) EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara, 2008. II. Final classification criteria. Ann Rheum Dis 69:798–806
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843
Coppo R, Camilla R, Amore A, Peruzzi L, Daprà V, Loiacono E, Vatrano S, Rollino C, Sepe V, Rampino T, Dal Canton A (2010) Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin Exp Immunol 159:73–81
Coppo R, Camilla R, Alfarano A, Balegno S, Mancuso D, Peruzzi L, Amore A, Dal Canton A, Sepe V, Tovo P (2009) Upregulation of the immunoproteasome in peripheral blood mononuclear cells of patients with IgA nephropathy. Kidney Int 75:536–541
Lin FJ, Jiang GR, Shan JP, Zhu C, Zou J, Wu XR (2012) Imbalance of regulatory T cells to Th17 cells in IgA nephropathy. Scand J Clin Lab Invest 72:221–229
Jung YO, Cho ML, Lee SY, Oh HJ, Park JS, Park MK, Park MJ, Ju JH, Kim SI, Kim HY, Park SH, Min JK (2009) Synergism of toll-like receptor 2 (TLR2), TLR4, and TLR6ligation on the production of tumor necrosis factor (TNF)-alpha in a spontaneous arthritis animal model of interleukin (IL)-1 receptor antagonist-deficient mice. Immunol Lett 123:138–143
Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, Chen WH, Ernst RK, Rossignol DP, Gusovsky F, Blanco JC, Vogel SN (2013) The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497:498–502
Mersmann J, Iskandar F, Latsch K, Habeck K, Sprunck V, Zimmermann R, Schumann RR, Zacharowski K, Koch A (2013) Attenuation of myocardial injury by HMGB1 blockade during ischemia/reperfusion is toll-like receptor 2-dependent. Mediators Inflamm 2013:174168
Savva A, Roger T (2013) Targeting Toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol 18:4–387
Niewerth D, Franke NE, Jansen G, Assaraf YG, van Meerloo J, Kirk CJ, Degenhardt J, Anderl J, Schimmer AD, Zweegman S, de Haas V, Horton TM, Kaspers GJ, Cloos J (2013) Higher ratio immune versus constitutive proteasome level as novel indicator of sensitivity of pediatric acute leukemia cells to proteasome inhibitors. Haematologica 98:1896–1904
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donadio, M.E., Loiacono, E., Peruzzi, L. et al. Toll-like receptors, immunoproteasome and regulatory T cells in children with Henoch–Schönlein purpura and primary IgA nephropathy. Pediatr Nephrol 29, 1545–1551 (2014). https://doi.org/10.1007/s00467-014-2807-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-2807-6