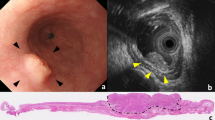
Abstract
Background and objectives
Neoadjuvant immunotherapy combined with chemotherapy or chemoradiotherapy has emerged as a promising approach in the treatment of esophageal cancer. However, there is a lack of comprehensive understanding regarding the clinical factors that can predict patient response to this therapy. The aim of this study was to develop a predictive model for assessing the efficacy of neoadjuvant immunotherapy in patients undergoing surgical treatment.
Methods
This study retrospectively enrolled 220 consecutive patients with preoperative immunotherapy combined chemotherapy or chemoradiotherapy. A logistic regression was used to evaluate the association between pathologic complete response (pCR) and endoscopic ultrasound parameters, constructing a predictive model for treatment response. Additional, overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan–Meier method, and Cox regression analyses were introduced to explore the associations between EUS factors after neoadjuvant immunotherapy.
Results
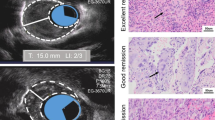
Logistic regression analysis identified that the significant predictors of pCR were treatment regimen, negative biopsy findings, RECIST assessment, endoscopic ultrasound responder, and downstaging in uN. A predictive model including above five variables was generated, and area under the curve was 0.840(95%CI 0.78–0.89), this nomogram was also adequately validated internally. In the cox regression analyses, EUS responder was found to be a significant predictor of overall survival with a hazard ratio (HR) of 0.38(95%CI 0.15–0.98), whereas only pCR status was a significant predictor of PFS (HR 0.80; 95%CI 0.01–0.60).
Conclusions
EUS responder can serve as a valuable predictor of the efficacy of adjuvant immunotherapy combined with chemotherapy or chemoradiotherapy, as well as of survival outcomes.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Bray F, Laversanne M, Sung H et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74(3):229–263. https://doi.org/10.3322/caac.21834
Herskovic A, Russell W, Liptay M, Fidler MJ, Al-Sarraf M (2012) Esophageal carcinoma advances in treatment results for locally advanced disease: review. Ann Oncol. https://doi.org/10.1093/annonc/mdr433
Lagergren J, Smyth E, Cunningham D, Lagergren P (2017) Oesophageal cancer. Lancet Lond Engl 390(10110):2383–2396. https://doi.org/10.1016/S0140-6736(17)31462-9
Ajani JA, D’Amico TA, Bentrem DJ et al (2019) Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 17(7):855–883. https://doi.org/10.6004/jnccn.2019.0033
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 16(1):1–24. https://doi.org/10.1007/s10388-018-0641-9
Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27(30):5062–5067. https://doi.org/10.1200/JCO.2009.22.2083
Li Q, Liu T, Ding Z (2022) Neoadjuvant immunotherapy for resectable esophageal cancer: a review. Front Immunol 13:1051841. https://doi.org/10.3389/fimmu.2022.1051841
Huang TX, Fu L (2019) The immune landscape of esophageal cancer. Cancer Commun (Lond) 39(1):79. https://doi.org/10.1186/s40880-019-0427-z
Yang Y, Tan L, Hu J et al (2022) Safety and efficacy of neoadjuvant treatment with immune checkpoint inhibitors in esophageal cancer: real-world multicenter retrospective study in China. Dis Esophagus 35(11):doac031. https://doi.org/10.1093/dote/doac031
Lv H, Zhang F, Huang C et al (2024) Survival outcomes of neoadjuvant immunochemotherapy versus chemotherapy for locally advanced esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 150(5):260. https://doi.org/10.1007/s00432-024-05793-4
Xu L, Wei XF, Li CJ et al (2022) After neoadjuvant immunochemotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. Front Immunol. https://doi.org/10.3389/fimmu.2022.1052542
Kudo T, Hamamoto Y, Kato K et al (2017) Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 18(5):631–639. https://doi.org/10.1016/S1470-2045(17)30181-X
Doi T, Piha-Paul SA, Jalal SI et al (2018) Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol 36(1):61–67. https://doi.org/10.1200/JCO.2017.74.9846
Chirieac LR, Swisher SG, Ajani JA et al (2005) Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 103(7):1347–1355. https://doi.org/10.1002/cncr.20916
Ancona E, Ruol A, Santi S et al (2001) Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 91(11):2165–2174
Shen J, Kong M, Yang H et al (2021) Pathological complete response after neoadjuvant treatment determines survival in esophageal squamous cell carcinoma patients (NEOCRTEC5010). Ann Transl Med 9(20):1516. https://doi.org/10.21037/atm-21-3331
Kalata S, Singh B, Graham N et al (2023) Epidemiology of postoperative complications after esophagectomy: implications for management. Ann Thorac Surg 116(6):1168–1175. https://doi.org/10.1016/j.athoracsur.2023.09.004
Farinella E, Safar A, Nasser HA et al (2016) Salvage esophagectomy after failure of definitive radiochemotherapy for esophageal cancer. J Surg Oncol 114(7):833–837. https://doi.org/10.1002/jso.24429
van der Wilk BJ, Eyck BM, Hofstetter WL et al (2022) Chemoradiotherapy followed by active surveillance versus standard esophagectomy for esophageal cancer: a systematic review and individual patient data meta-analysis. Ann Surg 275(3):467–476. https://doi.org/10.1097/SLA.0000000000004930
Sun JM, Shen L, Shah MA et al (2021) Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. The Lancet 398(10302):759–771. https://doi.org/10.1016/S0140-6736(21)01234-4
Chau I, Doki Y, Ajani JA et al (2021) Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): first results of the CheckMate 648 study. J Clin Oncol 39(18_suppl):LBA4001. https://doi.org/10.1200/JCO.2021.39.15_suppl.LBA4001
Luo H, Lu J, Bai Y et al (2021) Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 326(10):916. https://doi.org/10.1001/jama.2021.12836
Liu Z, Zhang Y, Ma N et al (2023) Progenitor-like exhausted SPRY1+CD8+ T cells potentiate responsiveness to neoadjuvant PD-1 blockade in esophageal squamous cell carcinoma. Cancer Cell. https://doi.org/10.1016/j.ccell.2023.09.011
Yang Y, Xin D, Wang H et al (2023) A novel predictor of pathologic complete response for neoadjuvant immunochemotherapy in resectable locally advanced esophageal squamous cell carcinoma. J Inflamm Res 16:1443–1455. https://doi.org/10.2147/JIR.S395231
Song XY, Liu J, Li HX et al (2023) Enhancing prediction for tumor pathologic response to neoadjuvant immunochemotherapy in locally advanced esophageal cancer by dynamic parameters from clinical assessments. Cancers 15(17):4377. https://doi.org/10.3390/cancers15174377
Vollenbrock SE, Voncken FEM, Van Dieren JM et al (2019) Diagnostic performance of MRI for assessment of response to neoadjuvant chemoradiotherapy in oesophageal cancer. Br J Surg 106(5):596–605. https://doi.org/10.1002/bjs.11094
Noordman BJ, Spaander MCW, Valkema R et al (2018) Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 19(7):965–974. https://doi.org/10.1016/S1470-2045(18)30201-8
Agarwal B, Swisher S, Ajani J et al (2004) Endoscopic ultrasound after preoperative chemoradiation can help identify patients who benefit maximally after surgical esophageal resection. Am J Gastroenterol 99(7):1258–1266. https://doi.org/10.1111/j.1572-0241.2004.30692.x
van der Bogt RD, Noordman BJ, Krishnadath KK et al (2019) Endoscopic ultrasound measurements for detection of residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. Endoscopy 51(4):326–332. https://doi.org/10.1055/a-0795-3220
Jost C, Binek J, Schuller JC et al (2010) Endosonographic radial tumor thickness after neoadjuvant chemoradiation therapy to predict response and survival in patients with locally advanced esophageal cancer: a prospective multicenter phase ll study by the Swiss Group for Clinical Cancer Research (SAKK 75/02). Gastrointest Endosc 71(7):1114–1121. https://doi.org/10.1016/j.gie.2009.12.015
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474. https://doi.org/10.1245/s10434-010-0985-4
Xie SH, Yang LT, Zhang H et al (2024) Adjuvant therapy provides no additional recurrence-free benefit for esophageal squamous cell carcinoma patients after neoadjuvant chemoimmunotherapy and surgery: a multi-center propensity score match study. Front Immunol 15:1332492. https://doi.org/10.3389/fimmu.2024.1332492
Wang H, Song C, Zhao X, Deng W, Dong J, Shen W (2023) Evaluation of neoadjuvant immunotherapy and traditional neoadjuvant therapy for resectable esophageal cancer: a systematic review and single-arm and network meta-analysis. Front Immunol 14:1170569. https://doi.org/10.3389/fimmu.2023.1170569
Li J, Hu H, Qin G et al (2024) Biomarkers of pathologic complete response to neoadjuvant immunotherapy in mismatch repair-deficient colorectal cancer. Clin Cancer Res 30(2):368–378. https://doi.org/10.1158/1078-0432.CCR-23-2213
Ji G, Yang Q, Wang S et al (2024) Single-cell profiling of response to neoadjuvant chemo-immunotherapy in surgically resectable esophageal squamous cell carcinoma. Genome Med 16(1):49. https://doi.org/10.1186/s13073-024-01320-9
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 1990 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Ratain MJ, Eckhardt SG (2004) Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol 22(22):4442–4445. https://doi.org/10.1200/JCO.2004.07.960
Westerterp M, van Westreenen HL, Reitsma JB et al (2005) Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy—systematic review. Radiology 236(3):841–851. https://doi.org/10.1148/radiol.2363041042
Ramon-Patino JL, Schmid S, Lau S et al (2022) iRECIST and atypical patterns of response to immuno-oncology drugs. J Immunother Cancer 10(6):e004849. https://doi.org/10.1136/jitc-2022-004849
Xu X, Zheng G, Zhang T, Zhao Y, Zheng Z (2019) Is pathologic tumor regression grade after neo-adjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? A cohort study evaluating tumor regression response. Cancer Chemother Pharmacol 84(3):635–646. https://doi.org/10.1007/s00280-019-03893-4
Jost C, Binek J, Schuller JC, Bauerfeind P, Metzger U et al (2010) Endosonographic radial tumor thickness after neoadjuvant chemoradiation therapy to predict response and survival in patients with locally advanced esophageal cancer: a prospective multicenter phase ll study by the Swiss Group for Clinical Cancer Research (SAKK 75/02). Gastrointest Endosc 71(7):1114–1121. https://doi.org/10.1016/j.gie.2009.12.015
Sethi D, Sen R, Parshad S, Khetarpal S, Garg M, Sen J (2012) Histopathologic changes following neoadjuvant chemotherapy in various malignancies. Int J Appl Basic Med Res 2(2):111–116. https://doi.org/10.4103/2229-516X.106353
Kimmey MB, Martin RW, Haggitt RC, Wang KY, Franklin DW, Silverstein FE (1989) Histologic correlates of gastrointestinal ultrasound images. Gastroenterology. https://doi.org/10.1016/0016-5085(89)91568-0
Shim CN, Song MK, Lee HS et al (2014) Prediction of survival by tumor area on endosonography after definitive chemoradiotherapy for locally advanced squamous cell carcinoma of the esophagus. Digestion 90(2):98–107. https://doi.org/10.1159/000365073
Hoibian S, Giovannini M, Autret A et al (2021) Preoperative EUS evaluation of the response to neoadjuvant therapy for gastric and esophagogastric junction cancer is correlated with survival: a single retrospective study of 97 patients. Endosc Ultrasound 10(2):103. https://doi.org/10.4103/EUS-D-20-00073
Mesenas S, Vu C, McStay M et al (2008) A large series, resection controlled study to assess the value of radial EUS in restaging gastroesophageal cancer following neoadjuvant chemotherapy. Dis Esophagus 21(1):37–42. https://doi.org/10.1111/j.1442-2050.2007.00731.x
Wu Y, Li J (2021) Change in maximal esophageal wall thickness provides prediction of survival and recurrence in patients with esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy and surgery. Cancer Manag Res. https://doi.org/10.2147/CMAR.S295646
Chen X, Chen X, Bao Y et al (2023) EUS-derived maximum tumor thickness and tumor shrinkage rate as independent prognostic factors in locally advanced esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. Endosc Ultrasound 12(4):369–376. https://doi.org/10.1097/eus.0000000000000008
van Rossum PSN, Goense L, Meziani J et al (2016) Endoscopic biopsy and EUS for the detection of pathologic complete response after neoadjuvant chemoradiotherapy in esophageal cancer: a systematic review and meta-analysis. Gastrointest Endosc 83(5):866–879. https://doi.org/10.1016/j.gie.2015.11.026
van der Wilk BJ, Eyck BM, Doukas M et al (2020) Residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer: locations undetected by endoscopic biopsies in the preSANO trial. Br J Surg 107(13):1791–1800. https://doi.org/10.1002/bjs.11760
Gu YM, Lyu SM, Shang QX et al (2022) Is tumor regression grade sufficient to predict survival in esophageal cancer with trimodal therapy? J Investig Surg 35(11–12):1818–1823. https://doi.org/10.1080/08941939.2022.2127036
Lopci E, Kauppi J, Lugaresi M et al (2019) Siewert type I and II oesophageal adenocarcinoma: sensitivity/specificity of computed tomography, positron emission tomography and endoscopic ultrasound for assessment of lymph node metastases in groups of thoracic and abdominal lymph node stations. Interact Cardiovasc Thorac Surg 28(4):518–525. https://doi.org/10.1093/icvts/ivy314
Tang H, Jiang D, Zhang S et al (2021) Residual tumor characteristics of esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg 162(6):1632–1641. https://doi.org/10.1016/j.jtcvs.2020.09.042
Chao YK, Chang Y, Yeh CJ, Chang HK, Tseng CK, Chuang WY (2016) Characterization of residual tumours at the primary site in patients with a near pathological complete response after neoadjuvant chemoradiotherapy for oesophageal cancer. Br J Surg 103(13):1874–1879. https://doi.org/10.1002/bjs.10293
Shapiro J, ten Kate FJW, van Hagen P, Biermann K, Wijnhoven BPL, van Lanschot JJB (2013) Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann Surg 258(5):678–688. https://doi.org/10.1097/SLA.0b013e3182a6191d. (discussion 688–689)
Bergeron EJ, Lin J, Chang AC, Orringer MB, Reddy RM (2014) Endoscopic ultrasound is inadequate to determine which T1/T2 esophageal tumors are candidates for endoluminal therapies. J Thorac Cardiovasc Surg 147(2):765–771. https://doi.org/10.1016/j.jtcvs.2013.10.003. (Discussion 771–773)
Rollins KE, Lucas E, Tewari N, James E, Hughes S, Catton JA (2015) PET-CT offers accurate assessment of tumour length in oesophageal malignancy. Eur J Radiol 84(2):195–200. https://doi.org/10.1016/j.ejrad.2014.10.014
Li B, Wang Y, Yu H et al (2023) Pattern of tumor regression after neoadjuvant chemoimmunotherapy for esophageal squamous cell carcinoma. J Thorac Dis 15(10):5517–5524. https://doi.org/10.21037/jtd-23-882
Funding
Nil.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Qiao-na Liu, Yu-fan Chen, Guang-yu Luo, and Xu Zhang have no conflicts of interest or financial ties to disclose.
Ethical approval
This was a retrospective study based on clinical data and was performed according to an institutional review board agreement under the authority of the Sun-Yat-sen University Cancer Hospital (Approval No. B2024-771-01). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s Human Research Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 19812 KB)
Supplement Figure 1. The ROC curve of EUS-derived maximum tumor thickness shrinkage rate.
Supplementary file2 (TIF 17687 KB)
Supplement Figure 2. The ROC curve of pretreatment EUS-derived maximum tumor thickness.
Supplementary file3 (TIF 16873 KB)
Supplement Figure 3. The ROC curve of post-treatment EUS-derived maximum tumor thickness.
Supplementary file4 (TIF 830 KB)
Supplement Figure 4. Kaplan–Meier curves depicting the assessment based by RECIST criteria and restaging in uN on PFS and OS for patients without adjuvant therapy.
Supplementary file5 (TIF 16728 KB)
Supplement Figure 5. Kaplan–Meier curves depicting the biopsy finding on PFS and OS for patients without adjuvant therapy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Qn., Chen, Yf., Luo, Gy. et al. Efficacy evaluation and prognostic prediction of endoscopic ultrasound for neoadjuvant immunotherapy in esophageal cancer. Surg Endosc (2025). https://doi.org/10.1007/s00464-025-11728-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00464-025-11728-y