Abstract
Introduction
Surgery is currently the only effective treatment for retroperitoneal tumors that do not involve any specific organ. The use of robots for removing both benign and malignant retroperitoneal tumors is considered safe and feasible. However, there is insufficient evidence to determine whether robotic retroperitoneal tumor resection (RMBRs) is superior to open retroperitoneal malignant resection (OMBRs). This study compares the short-term outcomes of robotic excision of benign and malignant retroperitoneal tumors with open excision of the same-sized tumors.
Methods
The study compared demographics and outcomes of patients who underwent robotic resection (n = 54) vs open resection (n = 54) of retroperitoneal tumors between March 2018 and December 2022. A 1:1 matching analysis was conducted to ensure a fair comparison.
Results
The study found that RBMRs resulted in reduced operative time (OT), estimated blood loss (EBM), and postoperative hospital stay (PSH) when compared to OBMRs. Additionally, RBMRs reduced EBL, PHS, and OT for patients with malignant tumor involvement in major vessels. No significant differences were found in tumor size, blood transfusion rate, and morbidity rate between the RBMRs and OBMRs groups.
Conclusion
When comparing RMBRs to OMBRs, it was observed that RMBR was associated with lower (EBL), shorter postoperative hospital stays (PHS), and reduced operative time (OT) in a specific group of patients with both benign and malignant tumors.
Similar content being viewed by others
Retroperitoneal tumors (RT) are mesenchymal tumors that can be benign or malignant. They develop in the retroperitoneal region and have no confirmed organ connection [1, 2]. Retroperitoneal tumors can be either benign or malignant, and they differ in various ways, such as their depth of attachment to significant vessels and operating space. The retroperitoneal great vessels are poorly understood, but they can potentially complicate surgical procedures and negatively impact treatment outcomes [3,4,5,6,7]. The retroperitoneum is a rare site for tumor growth, which can arise from various soft tissues and affect retroperitoneal organs. Retroperitoneal tumors can be benign or malignant, with malignant tumors further classified into those originating from epithelial tissues and those arising from non-epithelial tissues. Liposarcoma, leiomyosarcoma, and undifferentiated pleomorphic sarcoma are the most common subtypes. Certain types of sarcomas are more common in children, while diseases characterized by lymph node enlargement primarily affect adults. It is important to acquire a reliable diagnosis before considering surgery because the therapeutic techniques, including medical and surgical treatment, of malignant and benign tumors and the type and scope of surgery might differ significantly among the various kinds of tumors. A large number of benign and malignant tumors are eradicated through a laparotomy, which extends the hospital stay following surgery, postoperative agony, and delays healing [8,9,10,11]. A reliable diagnosis is crucial before surgery, as treatment options for malignant and benign tumors vary significantly. Robotic techniques are increasingly used in retroperitoneal surgeries due to limited space and advanced surgical equipment. These techniques have a shorter learning curve and result in better postoperative recovery, less blood loss, and shorter operation times compared to open surgeries [12] Robotic resection for tumors has limited published reports, mostly case studies. Despite advancements in robotic techniques, research on this topic is still limited [13,14,15,16,17,18]. Our study aims to compare the outcomes of robotic and open excision of retroperitoneal tumors. We hypothesize that robotic-assisted techniques will result in less operative time, bleeding, and postoperative complications, as well as minimal recurrence of tumors.
Patients and methods
We conducted a cross-sectional study to analyze the outcomes of surgical treatments performed between March 2018 and December 2022. During this period, we performed robotic resection on 104 patients and open resection on 68 patients. Before the surgery, experienced surgeons evaluated each patient to determine the best surgical option (robotic or open). All patients who were registered for the study were considered suitable candidates for both robotic and open surgery. They were given detailed information about the benefits and drawbacks of each surgical approach before making their final selection. There was no difference in tumor size between both groups. In this work, we applied propensity score matching to mitigate selection bias. Following the matching process, there were no obvious differences in demographic characteristics between the robotic and open groups. Patients provided their informed consent before the surgery and gave written consent for this study afterward. To compare the outcomes of robotic and open surgeries, we conducted a study with 1:1 propensity score analysis and telephonic interview were also performed. We matched 54 patients who had robotic surgery with 54 patients who had open surgery based on age, sex, BMI, ASA score, tumor size, and the procedure itself. The hospital ethical committee approved and registered this study.
Preoperative evaluation
Retroperitoneal tumors are detected through imaging and histological analysis. Ultrasound is the first method used, but its subjectivity is a drawback. Contrast-enhanced CT is preferred for assessing and staging primary retroperitoneal masses. Radiological indicators help establish a differential diagnosis, including the beak sign, phantom organ sign, embedded organ sign, and prominent feeding artery sign. CT angiography or 3D reconstruction is used for preoperative evaluation and surgical planning.
Inclusion and exclusion
To be eligible for inclusion, the patient must be between 18 and 82 years old, have a resect able retroperitoneal tumor (malignant or benign), and not have any medical conditions that would make anesthesia or surgery unsafe. Exclusion criteria include tumors bigger than 10 cm, no evidence of tumor involvement in the retroperitoneal organs, and severe cardiopulmonary or hepatorenal insufficiency. Surgery may still be considered if the tumor is adhering to major blood vessels.
Perioperative data
Data from preoperative and pathology records, along with baseline demographics, were collected. The following parameters were analyzed: operation time, estimated blood loss, blood transfusion, conversion to open surgery, postoperative complications, and PHS. Mortality and readmission rates within 1 year were also investigated. Pathology records were examined for tumor information.
Surgical technique and follow-up
RBMR surgeries were performed by an experienced team with over 1000 robotic procedures using the Da Vinci Xi Surgical System. The same team performed both robotic and open surgeries. For robotic resection the patient was positioned in the left lateral decubitus position with the right side elevated approximately 70 degrees during the surgery. They were under general anesthesia and continuously monitored with central venous and arterial pressure monitoring Fig. 1.
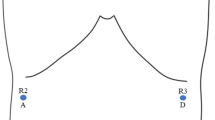
Port placement for robotic tumor excision. Trocar placement for the surgery. A six-trocar technique was applied for the surgery. One 12 mm trocar near the umbilicus was the designated camera port (point C). Camera port, robot arm port 1 (near the liver margin) and 2 (above the anterior superior iliac spine) formed approximate 120 angular degree. Assistant port A1and A2 were 8cm from the camera port. A 5 mm trocar under the liver margin near the midclavicular line was used as the assistant port for liver retraction (point A3)
To ensure an open approach, the patient is carefully placed in the right lateral Jackknife position at a 90-degree angle. The support for this is provided by both a beanbag and an axillary role. To ensure protection, the left arm is equipped with ample padding as well as an airplane-type retractor. It is important to secure the left arm such that the scapula rolls anteriorly. The incision will be made overlying the 11th or 12th intercostal space and extended toward the umbilicus. The position shifted in accordance with the tumor’s left or right location. Follow-up data was collected through outpatient departments and phone interviews. In the first year, patients underwent clinical examinations, blood tests, and imaging every 3 months.
Statistical analysis
Continuous data were reported as either mean SD or median based on their distributions. Categorical data were compared using the chi-square test. To reduce the impact of possible confounders and selection bias, propensity score analysis was used to match covariates such as age, sex, body mass index (BMI), ASA score, tumour size, and tumour involvement to major blood vessels between the two groups. A 1:1 nearest neighbour matching method was used to select individuals for each group. All statistical tests were performed using SPSS v22.0 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant.
Results
During the study period, 172 patients (104 RBMR and 68 OBMRs) had retroperitoneal benign and malignant tumors excision. Patients in the OBMRs group had a bigger tumors size and more blood transfusions than those in the RBMRs group before matching. In this propensity score matching trial, 54 patients RBMRs and 54 patients received OBMRs. After matching, there were not any significant differences in demographic variables between the two groups.
Pathological and postoperative outcome
Upon final histological inspection, it was found that the tumors in both groups were a mix of benign and malignant. Table 1 shows that both groups had similar pathology. Table 2 displays the perioperative results for both groups. The robotic group had significantly reduced surgical time (p = 0.004), lower estimated blood loss (p = 0.008), and shorter postoperative hospital stay (p = 0.001) compared to the open group. There was a significant postoperative complication, with 3 patients from each group experiencing lymphatic leakage and receiving treatment in accordance with hospital guidelines. Both groups had similar rates of blood transfusion (p = 0.7504). All patients underwent effective robotic removal of both benign and malignant tumors located in the retroperitoneal region, with no need to switch to laparotomy. The mortality rate in both groups was 1.8%. There were no patients who needed to undergo another operation or were readmitted to the hospital within 1 year.
Clinical outcomes of malignant tumors involvement the major vessels
Table 3 summarizes the characteristics of patients with malignant tumors involving major vessels before undergoing surgery. The data shows that both the Robotic and Open surgery groups had similar age, sex, BMI, and tumor size. They also had comparable rates of blood transfusion, illness, and mortality. The rates of positive surgical margin, local recurrence, adjuvant chemotherapy, radiotherapy, and target therapy were all similar between both groups. However, there were significant differences between the two groups in terms of estimated blood loss (EBL) (p = 0.0221), postoperative hospital stays (PHS) (p = 0.0001), and operation time (OT) (p = 0.0023).
Discussion
The findings of our study Robotic surgery group compared to the open surgery group. The robotic group showed significantly reduced PHS, intraoperative blood loss, and operating time. Increased accuracy and delicacy during manipulation may lessen surgical harm. All RBMRs in this study were performed exclusively utilizing the fully robotic method, without any need for conversion to open surgery. All patients who were registered for the study were considered suitable candidates for both robotic and open surgery. They were given detailed information about the benefits and drawbacks of each surgical approach before making their final selection. There was no difference in tumor size between both groups. In this work, we applied propensity score matching to mitigate selection bias. Following the matching process, there were no obvious differences in demographic characteristics between the robotic and open groups. Robotic surgery has gained popularity due to its ability to enhance manipulation capabilities, provide better visibility, and enable rapid bleeding management. It also decreases injury to the gastrointestinal system and expedites the recovery of gastrointestinal function as compared to open surgery [19,20,21,22,23,24]. Additionally, the results after surgery are consistently getting better and it is widely accepted. Robotic surgery is rapidly becoming the preferred method in urology experiencing substantial growth in industrialized countries. That they have exceeded the outcomes of traditional open surgery for most treatments [25]. Robotic resection techniques are safe and feasible for removing retroperitoneal tumors, including large tumors or those attached to major blood arteries, with no significant negative impact on perioperative outcomes [26]. Due to the greater probability of malignant retroperitoneal tumors invading major vasculature, we further categorized the patients based on the tumor type. Our findings indicate that robotic surgery considerably reduced estimated blood loss (EBL) and postoperative hospital stay (PHS) in these patients as compared to open surgery patients. The enhanced dexterity and visualization provided by the robotic technology can be credited for its positive impact on tumor dissection, tissue mobilization, and vascular control. Retrospective and randomized controlled trials provide ample evidence indicating that use of minimally invasive techniques for treating early-stage lung cancer, bladder cancer, cervical cancer offers numerous clinical benefits in terms of perioperative outcomes compared to open surgery [27,28,29]. Robotic retroperitoneal surgery resulted in significantly less blood loss than open retroperitoneal surgery for both benign and malignant cases. Traditional open or laparoscopic surgical procedures, such as partial nephrectomy, radical nephrectomy, retroperitoneal lymph node dissection, nephroureterectomy, and adrenalectomy, are now frequently performed with robot assistance. Robot-assisted retroperitoneal oncological surgery is continually evolving, with a special focus on preserving oncological and functional results while reducing surgical invasiveness [16, 30]. Additionally, there was no significant difference observed in the rates of postoperative morbidity between RMBR and OMBR. This finding implies that robotic resection of malignant tumors measuring no larger than 10 cm seems to be technically safe and efficacious. Robotic surgery resulted in faster recovery than open surgery in both our study and the study conducted by Giulianotti PC et al. Patients who underwent robotic surgery had early bowel function recovery, minimal analgesic needs, and did not require blood transfusions. All patients resumed a normal diet by day two, and most experienced flatulence and bowel movement within the first day. Robotic surgery is a safe option for benign and malignant tumor resection [31]. In this trial, 172 patients were studied, out of which 104 underwent entirely robotic treatment without requiring open surgery. The robotic group showed a significantly shorter postoperative stay when compared to the open group in our study. Our study demonstrated statistically significant differences in operation times and shorter recovery times for robot-assisted retroperitoneal tumor removal when compared to the open retroperitoneal tumor resection group. Robotic surgery offers improved dexterity, intuitive instrument handling, tremor reduction, motion scaling, and superior 3D visualization [32, 33]. As surgeons gain more experience in operating robotic systems, they tend to become more efficient, which results in shorter operative times. Robotic systems provide better precision, dexterity, and visualization when compared to traditional open surgery, which allows for more accurate and efficient complex procedures. However, there may be a learning curve for surgeons and their teams when adopting robotic surgery. As the experience of surgeons increases, the time taken for operations tends to decrease. Moreover, this study has shown that there were statistically significant differences in the blood transfusion rates between the two patient groups.
Limitations
This study has several limitations, including a relatively small sample size, limited evaluation of long-term outcomes, and lack of information about the surgeons' experience level with robotic surgery. Additionally, the study did not evaluate the cost-effectiveness of robotic surgery or potential confounding factors that may impact the outcomes. Future studies should address these limitations by conducting larger-scale investigations that evaluate the long-term effects of robotic surgery on patients, assess the cost-effectiveness of the procedure, and account for potential confounding factors. Furthermore, future studies should evaluate the feasibility and safety of robotic surgery for retroperitoneal tumors in a variety of settings and patient populations. By addressing these limitations and exploring future perspectives, researchers can continue to improve the outcomes and accessibility of surgical treatments for retroperitoneal tumors.
Conclusion
In conclusion, our findings show that RMBRs are as safe and effective as OMBRs in patients with retroperitoneal benign and malignant tumors. With the benefits of the robotic approach, RMBRs may offer significant improvements over OMBRs, reducing OT, EBL and PHS in patients even for tumors involving major vessels.
Data availability
Researchers who make a valid request to the corresponding author will be given access to the data.
References
Rutkowski P, Lugowska I (2014) Follow-up in soft tissue sarcomas. Memo 7(2):92–96
Berelavichus S, Kriger A, Kaldarov A, Panteleev V, Raevskaya M (2021) Robotic surgery in treatment of retroperitoneal tumors. Comparative single center study. J Robotic Surg 15(3):363–367
Maruyama T, Sugamoto Y, Miyagishima D, Fukunaga T, Tasaki K, Takeshita N et al (2015) Laparoscopic resection of a retroperitoneal schwannoma located in the hepatic hilus. Surg Case Rep 1(1):18
Cadeddu MO, Mamazza J, Schlachta CM, Seshadri PA, Poulin EC (2001) Laparoscopic excision of retroperitoneal tumors: technique and review of the laparoscopic experience. Surg Laparosc Endosc Percutan Tech 11(2):144–147
Tip SWM, Lee YT, Tang PH, Chang KTE, Soh SY, Tan AM et al (2019) Retroperitoneal tumors and congenital variations in vascular anatomy of retroperitoneal great vessels. J Pediatr Surg 54(10):2112–2116
Ahn KS, Han HS, Yoon YS, Kim HH, Lee TS, Kang SB et al (2011) Laparoscopic resection of nonadrenal retroperitoneal tumors. Arch Surg 146(2):162–167
Improta L, Tzanis D, Bouhadiba T, Abdelhafidh K, Bonvalot S (2020) Overview of primary adult retroperitoneal tumours. Eur J Surg Oncol 46(9):1573–1579
Osman S, Lehnert BE, Elojeimy S, Cruite I, Mannelli L, Bhargava P et al (2013) A comprehensive review of the retroperitoneal anatomy, neoplasms, and pattern of disease spread. Curr Probl Diagn Radiol 42(5):191–208
Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD (2015) Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging 40(6):1887–1903
Neville A, Herts BR (2004) CT characteristics of primary retroperitoneal neoplasms. Crit Rev Comput Tomogr 45(4):247–270
Rosenberg AE (2013) WHO classification of soft tissue and bone, fourth edition: summary and commentary. Curr Opin Oncol 25(5):571–573
Ludwig WW, Gorin MA, Pierorazio PM, Allaf ME (2017) Frontiers in robot-assisted retroperitoneal oncological surgery. Nat Rev Urol 14(12):731–741
Kriger AG, Berelavichus SV, Son AI, Gorin DS, Akhtanin EA, Kaldarov AR et al (2017) Surgical treatment of retroperitoneal masses. Khirurgiia (Mosk) 1:15–26
Cochetti G, Barillaro F, Boni A, Del Zingaro M, Ettore M (2014) Robot assisted laparoscopic excision of a paraganglioma: new therapeutic approach. Int Braz J Urol 40(2):279–280
Ragu R, Blanchard C, Meurette G (2017) Robotic excision of large retroperitoneal schwannoma (with video). J Visc Surg 154(4):297–299
Xia L, Xu T, Wang X, Qin L, Zhang X, Zhang X et al (2016) Robot-assisted laparoscopic resection of large retroperitoneal paraganglioma-initial experience from China. Int J Med Robot 12(4):686–693
Lehrfeld T, Natale R, Sharma S, Mendoza PJ, Schwab Ii CW, Lee DI (2010) Robot-assisted excision of a retroperitoneal mass between the left renal artery and vein. Jsls 14(3):447–449
Perrin H, Brunner P, Ortega JC, Mercier B, Clement N, Robino C et al (2017) Robotic resection of an obturator schwannoma with preservation of normal nerve fascicles and function. J Robot Surg 11(4):479–483
Darzi SA, Munz Y (2004) The impact of minimally invasive surgical techniques. Annu Rev Med 55:223–237
Corcione F, Esposito C, Cuccurullo D, Settembre A, Miranda N, Amato F et al (2005) Advantages and limits of robot-assisted laparoscopic surgery: preliminary experience. Surg Endosc 19(1):117–119
Garcia-Ruiz A, Gagner M, Miller JH, Steiner CP, Hahn JF (1998) Manual vs robotically assisted laparoscopic surgery in the performance of basic manipulation and suturing tasks. Arch Surg 133(9):957–961
Jin YM, Liu SS, Chen J, Chen YN, Ren CC (2018) Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PLoS ONE 13(3):e0193033
Lin Y, Zhang Y, Luo L, Zhang X (2021) Clinical effect of robot-assisted radical cystectomy in bladder cancer. Am J Transl Res 13(9):10545–10553
Lairmore TC, Folek J, Govednik CM, Snyder SK (2016) Improving Minimally invasive adrenalectomy: selection of optimal approach and comparison of outcomes. World J Surg 40(7):1625–1631
Mikhail D, Sarcona J, Mekhail M, Richstone L (2020) Urologic robotic surgery. Surg Clin North Am 100(2):361–378
Liu Q, Gao Y, Zhao Z, Zhao G, Liu R, Lau WY (2018) Robotic resection of benign nonadrenal retroperitoneal tumors: a consecutive case series. Int J Surg 55:188–192
Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R et al (2018) Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 379(20):1895–1904
Demmy TL, Curtis JJ (1999) Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 68(1):194–200
Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB (2016) Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 17(6):836–844
Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P et al (2010) Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 24(7):1646–1657
Li X, Xiao S, Yu Y, Liu W, Xi H, Wang G et al (2023) Robotic-assisted laparoscopic adrenalectomy (RARLA): what advantages and disadvantages compared to retroperitoneal laparoscopic adrenalectomy (RLA)? Front Endocrinol (Lausanne) 14:1145820
Herron DM, Marohn M (2008) A consensus document on robotic surgery. Surg Endosc 22(2):313–325
Khan MS, Elhage O, Challacombe B, Rimington P, Murphy D, Dasgupta P (2011) Analysis of early complications of robotic-assisted radical cystectomy using a standardized reporting system. Urology 77(2):357–362
Acknowledgements
Not applicable' for that section.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
M.S collected data, performed statistical analysis, and authored original drafts of introduction, discussion, and results. K.A.A wrote the abstract, limitations, conclusion, and made necessary corrections. Y.C provided supervision, corrected data issues. R.H reviewed findings and made revisions. Y.L reviewed results and offered feedback. Z.Z.X interpreted statistical analysis. Z.W served as corresponding author. H.H reviewed the study. Final approval of the manuscript: All authors.
Corresponding author
Ethics declarations
Disclosures
Each author (Manan Sulaiman, Khan Akhtar Ali, Yang chunguang, Rubina Hashim, Yang Luan, Ze Zhong Xiong, Hui Huang and Zhihua Wang) have no conflicts of interest or financial ties to disclose. All the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This study (RCT) is registered under registration number 2019CR101, Informed consent was obtained from all the patients. The ethics committees of Hospital approved the study. The authors declare that the methods carried out in this study were by the relevant guidelines and regulations.
Consent for publication
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (mp4 2239 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sulaiman, M., Ali, K.A., Chunguang, Y. et al. Open versus robot-assisted retroperitoneal tumors resection involving inferior vena cava, abdominal aorta, and renal hilum: a comparative study. Surg Endosc (2024). https://doi.org/10.1007/s00464-024-10848-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00464-024-10848-1