Abstract
Background
Endometriosis is a chronic condition affecting 6–10% of women of reproductive age, with endometriosis-related pain and infertility being the leading symptoms. Currently, the gold standard treatment approach to surgery is conventional laparoscopy (CL); however, the increasing availability of robot-assisted surgery is projected as a competitor of CL. This study aimed to compare the perioperative outcomes of robot-assisted laparoscopy (RAL) and CL in endometriosis surgery.
Objectives
We aimed to compare the effectiveness and safety of these two procedures.
Methods
A systematic search was conducted in three medical databases. Studies investigating different perioperative outcomes of endometriosis-related surgeries were included. Results are presented as odds ratios (OR) or mean differences (MD) with 95% confidence intervals (CI).
Results
Our search yielded 2,014 records, of which 13 were eligible for data extraction. No significant differences were detected between the CL and RAL groups in terms of intraoperative complications (OR = 1.07, CI 0.43–2.63), postoperative complications (OR = 1.3, CI 0.73–2.32), number of conversions to open surgery (OR = 1.34, CI 0.76–2.37), length of hospital stays (MD = 0.12, CI 0.33–0.57), blood loss (MD = 16.73, CI 4.18–37.63) or number of rehospitalizations (OR = 0.95, CI 0.13–6.75). In terms of operative times (MD = 28.09 min, CI 11.59–44.59) and operating room times (MD = 51.39 min, CI 15.07–87.72;), the RAL technique remained inferior.
Conclusion
RAL does not have statistically demonstrable advantages over CL in terms of perioperative outcomes for endometriosis-related surgery.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endometriosis is an estrogen-dependent benign gynecological disorder associated with pelvic pain and infertility. Globally, approximately 70 million women of reproductive age suffer from various forms of endometriosis [1]. It is characterized by the presence of functioning endometrium-like tissue outside the uterine cavity, which induces an inflammatory response [2]. The leading complaints of endometriosis are dysmenorrhea, dyspareunia, dysuria, and dyschezia, accompanied by infertility. Several therapeutic options, including medications, surgical interventions, and non-medical management strategies, aim to reduce pain-related symptoms and restore fertility [3]. Surgical approaches consist mainly of minimally invasive techniques. Their advantages include lower rates of complications, such as shorter hospital stays, reduced trauma, and lower chances of infections compared to open surgeries [4]. Conventional laparoscopic surgery is considered as the standard of care for the above reasons; however, its limitations include 2-dimensional (2D) visualization, ergonomic challenges for the surgeon, and limited instrument range [5]. As more advanced techniques such as robot-assisted laparoscopy (RAL) are becoming more and more prevalent, the above limitations of laparoscopy are expected to be overcome.
RAL has the advantages of minimally invasive surgery, but also has other benefits. On the surgical side, robot-assisted surgery, using 3D technology, offers better visualization of the surgical site, instrumentation facilitates seven degrees of freedom, permits tremor-free handling, and reduces work fatigue while also having a shortened learning curve compared to laparoscopic surgery [4]. Previous studies have shown that RAL has clinically relevant advantages in numerous other surgical areas (e.g., rectal cancer resection and distal pancreatectomy) [5, 6]. Advantages reported in the outcome of robot-assisted operations compared to CL include reduced postoperative pain and blood loss [7]. However, the two main disadvantages of the robot include the absence of tactile feedback and the high cost of installing and maintaining machinery.
Although the benefits of RAL have been demonstrated in several surgical fields, its benefits over CL in endometriosis have not yet been investigated. Therefore, we aimed to compare the effectiveness and safety of these two procedures.
Materials and methods
Our systematic review and meta-analysis was reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 Statement. (Supplementary Table 1.) This study followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3 [8]. The review protocol was registered on PROSPERO (York, UK) under the registration number CRD42023397045.
Literature search and eligibility criteria
A systematic literature search was performed using three medical databases, MEDLINE (via PubMed), Cochrane Library (CENTRAL), and Embase on February 15, 2023. The main domains of the search key were endometriosis, robot-assisted surgery, and laparoscopy. The full search key is presented in Supplementary Table S5. Case reports, case series, conference abstracts, trial protocols, letters, and reviews were excluded. No language or other restrictions were imposed.
Papers were eligible if they conformed to our PICO (Population, Intervention, Comparison, Outcome) framework. Articles on premenopausal women who underwent surgery for endometriosis (P) were included. The diagnosis of endometriosis was based on either of the following: clinical symptoms, imaging techniques, laparoscopic findings, or histology. The included studies required robot-assisted surgery as an intervention (I) compared to the conventional laparoscopic approach (C). Our outcomes were different perioperative outcomes: intra-, and postoperative complications, operating room time, operative time, anesthesia time, number of recurrences, estimated blood loss, and length of hospital stay following surgery (O). An important criterion was that the articles had to define the outcomes mentioned above in the same way for the two surgical approaches. Detailed exclusion and inclusion criteria are presented in Supplementary Table S2.
Study selection and data collection
EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) was used for duplicate removal, rayyan.ai for title-abstract selection, and EndNote X9 for full-text selection. At every level of selection, two independent authors (ÁC, DPK) screened the publications, and disagreements were resolved by a third author (ÁJ).
Two authors (ÁC, DPK) independently extracted data into a predefined Excel spreadsheet (Office 365, Microsoft, Redmond, WA, USA). The following data were extracted from each eligible article: first author, year of publication, study type, study location, number of centers involved, study design, demographic data (sample size, age, body mass index (BMI), presence of infertility, previous surgeries, details of procedures, and number of surgeons performing the operations) and data for the outcomes for statistical analysis. A third reviewer (ÁJ) resolved the discrepancies. Cohen's kappa coefficient (κ) was calculated after each step to measure interrater reliability [9].
Quality assessment and quality of evidence
The quality of the outcomes was assessed separately by two reviewers (ÁC, ÁJ) using the risk of bias tool Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I) for non-randomized- and RoB 2 for randomized trials. Any disagreements were resolved by a third reviewer (DPK). The VISualization (Robvis) tool was used to visualize the results [10].
The recommendations of the "Grades of Recommendation, Assessment, Development, and Evaluation (GRADE)" workgroup were followed to evaluate the quality of evidence [11].
Data synthesis and analysis
The odds ratio with 95% CI was used to measure the effect of intra- and postoperative complications, whereas mean differences (MDs) were used for outcomes measuring operation durations. To calculate the odds ratio, the total number of patients in each group and those with the event of interest were extracted from each study, whereas we used the between-group mean differences and SDs to calculate the effect measure for continuous outcomes. Raw data from the selected studies were pooled using a random-effects model with the Mantel–Haenszel method and the Hartung–Knapp adjustment [12, 13]. To estimate τ2, we used the Paule-Mandel method and the Q profile method to calculate the confidence interval of τ2. A funnel plot of the logarithm of effect size and comparison with the standard error for each trial was used to evaluate publication bias. Statistical heterogeneity across trials was assessed by means of the Cochrane Q test, and the I2 statistic values. Outlier and influence analyses were carried out following the recommendations of Harrer et al. and Viechtbauer and Cheung [13, 14]. Forest plots were used to graphically summarize results. Where applicable, we reported the prediction intervals (i.e., the expected range of effects of future studies) of results as recommended by IntHout et al. 2016 [15]. All analyses were carried out using R 4.1.3, the packages ‘meta’ and ‘dmetar’ [16,17,18].
Results
Search and selection
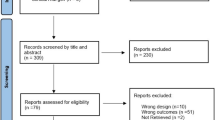
A total of 2,014 studies were identified. After removing 1,382 duplicates, we found 632 eligible studies by title abstract, of which 38 were eligible for full-text selection. Finally, the data from 13 articles were extracted (Fig. 1).
Basic characteristics
Of these articles, one was a randomized controlled trial (RCT), four were prospective, and eight were retrospective cohort studies, published between 2010 and 2022. Most of them implemented multiport laparoscopic surgeries, with two exceptions, which applied the single port technique [19, 20]. Ten articles contained information on the type of robot used, which was the da Vinci type in all cases, and also, ten studies reported on the number of surgeons performing the surgeries, ranging from one to five. Ten articles also evaluated the experience of the surgeons as the expertise of the surgeons based on subjective reports or metric scales, but all surgeons were experts.
The CL group included 1,009 patients and the RAL group 1,012. The baseline data are summarized in Table 1. The mean ages and BMIs of the two groups were similar, as well as the severity of endometriosis operated on. However, the latter was different between articles, five articles included only severe cases of endometriosis, but this did not provide a basis for selection [21,22,23,24,25]. In terms of study designs, the study by Soto et al., the only RCT, represented the highest quality, although its primary limitation was the inclusion of suspected endometriosis cases as well [26]. Data on anesthesia time and postoperative recurrence were not available for analysis. Furthermore, the examined articles did not provide data on the impact of interventions on the quality of life.
Complications
First, we examined intraoperative complications, which were evaluated in 11 articles, without specifying the type of complications, only their number. The two groups showed no difference (OR = 1.07, CI 0.43–2.63) in the number of complications (Fig. 2). The relative frequency of complications in the RAL group was 1.21% and 1.32% in the CL group.
Postoperative complications were also investigated in 11 articles without specifying the exact timing or type of complications, only their number. No differences in postoperative complications were detected between the CL and RAL groups (OR = 1.3, CI 0.73–2.32) (Fig. 3). The relative frequency of postoperative complications in the RAL group was 7.96%, and 10.07% in the CL group. Furthermore, four articles categorized these complications based on the Clavien-Dindo classification, with results similar to those of previous studies (Supplementary Figures S10-12).
Ten articles investigated the number of laparotomy conversions to open surgery. Neither CL nor RAL had clinically relevant, higher conversion rates (OR = 1.34, CI 0.76–2.37) (Supplementary Fig. S13). The relative frequencies of conversions in the RAL group were 0.74% and 0.49% in the CL group, respectively.
Three articles evaluated the number of rehospitalizations. No significant difference was observed between the two procedures (OR = 0.95, CI 0.13–6.75) (Supplementary Figure S14).
Estimated blood loss
Eleven articles examined the estimated blood loss in milliliters during surgery. One article (Lee 2020) reported blood loss in grams of hemoglobin per deciliter; therefore, these data were omitted from the analysis. Approximately 16 ml more blood was lost during RAL surgeries (MD = 16.73, CI 4.18–37.63) (Supplementary Fig. S15) However, this finding was neither clinically relevant nor statistically significant.
Length of the procedures
Twelve articles evaluated the operative time, measured in minutes from skin incision to wound closure. For the robot-assisted technique, time included the times for both docking and undocking. The operative times showed that the robot-assisted technique took almost half an hour (MD = 28.09, CI 11.59–44.59) (Fig. 4) longer compared to CL. This result was clinically relevant and statistically significant.
Three studies examined the time spent in the operating room, measured in minutes, from patient arrival to departure from the operating room. Docking and undocking times were also included for RAL. Similarly, these results favored CL (MD = 51.39, CI 15.07–87.72) (Fig. 5), being clinically relevant and statistically significant.
Length of hospital stay
Eight studies analyzed the number of days spent in hospital following surgery (MD = 0.12, CI 0.33–0.57) (Supplementary Fig. S16), showing neither clinically relevant nor statistically significant differences.
Quality and risk of bias assessment
The risk of bias was assessed using the ROBINS-I tool for observational studies. Most articles were rated as "moderate." Three articles lacked information on how patients were assigned to the RAL or CL groups; thus, two received a "serious" risk for bias classification, and one a "critical" rating because information on the number of physicians performing the operations was missing. One additional article received a "serious" rating because it did not include information on the number of surgeons involved. (Supplementary Figs. S1-8).
For the RCT, the risk of bias was assessed using the RoB 2 tool, resulting in a low-risk rating. (Supplementary Fig. S9).
GRADEpro was used for quality control with moderate rating results for all evaluated outcomes. (Supplementary Table S3).
Intra- and postoperative complications, the number of rehospitalizations, and the number of conversions had a low heterogeneity. However, heterogeneity was high for operative times, estimated blood loss, and length of hospital stay. The high heterogeneity could be due to the differences in the severity of endometriosis. Unlike for operative time, low heterogeneity was observed for operating room time, probably due to the inclusion of endometriosis with the same severity.
Discussion
This systematic review and meta-analysis identified 13 studies that compared RAL with CL in terms of perioperative outcomes of endometriosis surgery. The quantitative synthesis of our findings confirmed that RAL had no numerical advantages over CL in the aspects studied. Moreover, we found it to be inferior in terms of operating room and operative times. Subgroup analysis based on the pattern of endometriosis was not feasible with the available data.
In addition to previous studies, we examined operating room time as a new outcome [27,28,29]. However, we obtained similar results for all other perioperative outcomes. Our results did not show the expected benefit of RAL over CL in terms of intra- and postoperative complications, estimated blood loss, number of rehospitalizations, or days spent in hospital, and we observed even longer operative times by approximately 30 min. The latter can be attributed to an average docking time of approximately 18.2 min [30]. Operating time was found to be the most significant factor associated with postoperative complications and length of postoperative hospital stay. Magrina et al. found that for every additional 60 min of operating time, the odds of postoperative complications and prolonged hospital stay increased the chances by 57% and 103%, respectively [24]. This is partly explained by the disproportionate distribution of patients. In some articles, more radical procedures (e.g., endometriosis surgery with concomitant hysterectomy) were performed and patients with more advanced endometriosis were operated on with RAL according to the revised American Society for Reproductive Medicine (rASRM) staging. This might indicate that the surgeon favored a robotic approach and might have added bias to the results, contributing to differences in operating times [24, 31]. However, we did not find significant differences in rASM classification between the two approaches. (Supplementary Figures S17-S20) On the other hand, the experience of Nezhat suggests that procedures for the treatment of severe disease require multiple camera and instrument exchanges, making CL easier to perform [23].
It should be noted that the only RCT conducted by Soto 2017 found the mean operative time and blood loss within the range of time and volumes previously reported by other non-randomized studies. This suggests that their findings are unlikely to be related to patient selection and the experience of the surgeons or the team with various platforms [32]. Not surprisingly, in the articles describing more severe cases (e.g., bowel (deep infiltrating endometriosis (DIE), rASRM stage III/IV.), an even longer operative times could be observed compared to the mean difference we reported. Similar to the operative time, we obtained a significant difference of approximately 50 min in the operating room time. This difference could also be attributed to the necessary preparation procedures of the robot in addition to factors described influencing the length of the surgery time.
Intraoperative complications were crucial in determining intra- and postoperative outcomes, such as operative time (thus, operating room time), expected blood loss, the likelihood of conversion to open surgery, days spent in hospital, and postoperative complications. Most studies showed relatively low numbers of intraoperative and postoperative complications, indicating that both methods were safe and neither seemed to be superior in terms of complication rates. It should be noted that Carpentier et al. only operated on bladder DIE, and the relative frequency of postoperative complications in the RAL group was 60% versus 36% in the CL group [32]. Conversion to open surgery depended on several factors, including the previous abdominal surgeries of the patient and unexpected technical events, among other factors. However, the level of experience of the surgeon was a factor that needs to be highlighted.
Other meta-analyses had also been conducted on this topic; however, due to the low number of cases and methodological problems, we considered it necessary to conduct another meta-analysis. In the meta‐analysis by Chen (2016), RAL was compared with CL for endometriosis surgery; no difference was found in most aspects, except for operating time [27]. In 2020, Restainato et al. and in 2018, Balla et al. performed a meta-analysis and found no difference in the operating time or complication rates between robot-assisted and conventional laparoscopy. However, in the latter study, in which all patients underwent colorectal resection due to endometriosis, only a small fraction (1.7%) of the procedures were performed with RAL. Moreover, complications were not evaluated separately for RAL and CL [28, 29].
Our study showed that RAL did not offer a quantifiable advantage in the day-to-day surgical management of patients with endometriosis. However, the reality is more nuanced; an important finding is that longer operative time has been correlated with increased overall costs strongly associated with the robotic platform [23]. As for costs, no data were found for endometriosis surgeries; however, such data were reported in closely related areas. A database study of 36,188 patients showed that robotic hysterectomy was more expensive than laparoscopic hysterectomy ($9,640 vs. $6,973, P < 0.01). In gynecological oncology, for endometrial or cervical cancer, the extra cost of using RAL was €1,456 per intervention [33].
Le Gac et al. mentioned earlier that the learning curve of robotic surgery in general could have influenced docking and operative times, as well as the complication rate [22]. The articles by Lee et al. and Terzi et al. demonstrated that the learning curves of RAL and CL differed significantly. For RAL surgeries, the operation time for hysterectomy could be reduced after 23 surgeries of the same type, whereas for CL, 75 surgeries were required [34, 35].
However, experts of both RAL and CL have experienced the convenience of using RAL, as it provides comfort and increased precision in the operating technique. The RAL offers better visualization of the surgical site using 3D technology and 15 times magnification. Also, from an ergonomic point of view, instruments that mimic the movement of human hands, wrists, and fingers allow an extensive range of motion that is more precise than natural hand and wrist movements. Owing to the robotic arms, the sustained maintenance of positions demanding substantial force does not precipitate deleterious consequences or substantial fatigue. CL also has indisputable advantages, such as haptic tissue feedback, which, for example, is particularly beneficial for establishing the pathological-healthy border during the excision of a DIE nodule. Also, the esthetic effect is highlighted mainly from the patients' point of view. For CL, both the location of the ports on the trunk and the smaller diameter of the trocars are options preferred by patients. Robot-assisted surgery appears to have fewer negative cognitive and musculoskeletal impacts on surgeons than CL [36]. In 2021, Sers et al. found that performing laparoscopic surgery on patients, especially with high BMIs, increased the prevalence of non-neutral postures and could have further increased the risk of musculoskeletal disorders in surgeons [37]. However, to date, no studies have investigated the more serious, long-term, irreversible effects of CL on health, such as the potential development of knee and hip joint impairment. Current recommendations for the use of RAL in the surgical treatment of endometriosis vary depending on several factors, including the individual circumstances of the patients, the expertise of the surgeon, and the availability of resources and equipment. In 2013, the American Association of Gynecologic Laparoscopists (AAGL) recommended that RAL should not replace CL or vaginal procedures for women who could otherwise undergo CL or vaginal surgery for benign gynecologic diseases [38]. On the basis of the guidelines of the Danish Health Authority, RAL hysterectomy should only be preferred over CL hysterectomy after careful consideration because its beneficial effect is uncertain due to longer operating time [39]. Especially, with regard to advanced stage endometriosis, RAL is a possible first-line approach for the surgical treatment of bowel DIE [23, 40]. Furthermore, Lee et al. conclude that robot-assisted cystectomy in bilateral ovarian endometrioma is better than the laparoscopic approach for preserving ovarian function [20]. The decision to use robot-assisted laparoscopy for the treatment of endometriosis should be made on a case-by-case basis, taking into account the specific needs and circumstances of the patient as well as the experience and skill of the surgeon. Patients are advised to consult their healthcare providers to determine the most appropriate treatment approach for their individual situations. It is essential to highlight that this was only a snapshot. As time passes, expert surgeons who have spent most of their lives with laparoscopy will spend more time with the robot-assisted technique and could produce entirely new results.
Strengths and limitation
We followed our rigorous protocol, which had been registered in advance. Our investigation covered a long study period, with a high number of cases. Although there had been previous meta-analyses on the topic, we were able to include more articles than the latest one from 2020. Compared with previous meta-analyses, our review examined operating room time as a new outcome.
As for the limitations of our analysis, most articles were retrospective studies, and only one RCT was included. In most of the included articles, patient selection was based on the availability of a robotic room. Also, some articles performed only certain organ-specific interventions and operated only on a specific severity of endometriosis, thus not representing the full range. Furthermore, it is imperative to underscore that the same author has contributed to some of the selected articles.
Conclusion
On the basis of our study, in most aspects, RAL seems to be equivalent to CL; however, in terms of time efficiency, it is inferior in the treatment of endometriosis.
Implications for practice and research
Translating scientific knowledge for the benefit of patients has crucial importance [41, 42]. Our research suggests that in general practice, CL should be the first choice in the surgical treatment of patients with endometriosis. It is also financially advantageous, but is generally not considered in studies and has yet to be explored, especially when weighed against the cost of training a new surgeon and the lengthy learning curve compared to RAL.
However, RAL has practical advantages over CL, which has been poorly studied. From a surgical point of view, it has advantages in terms of posture, resulting in fewer orthopedics-related problems, and from the point of view of the patient, the higher magnification and better maneuverability may lead to more precise treatment. Further studies are needed to explore the medico-economic aspects of these two interventions.
Data availability
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
References
González-Mesa E, Moya-Bejarano D, Butrón-Hinojo CA, Marín-Sánchez P, Blasco-Alonso M, Jimenez-López JS, Villegas-Muñoz E, Lubián-López DM (2021) Correlates of sexual function in a sample of Spanish women with endometriosis. J Clin Med 10(21):4957. https://doi.org/10.3390/jcm10214957
Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M, Wei J (2019) Endometriosis. Endocr Rev 40(4):1048–1079. https://doi.org/10.1210/er.2018-00242
Samy A, Taher A, Sileem SA, Abdelhakim AM, Fathi M, Haggag H, Ashour K, Ahmed SA, Shareef MA, AlAmodi AA, Keshta NHA, Shatat HBAE, Salah DM, Ali AS, El Kattan EAM, Elsherbini M (2021) Medical therapy options for endometriosis related pain, which is better? A systematic review and network meta-analysis of randomized controlled trials. J Gynecol Obstet Hum Reprod. 50(1):101798. https://doi.org/10.1016/j.jogoh.2020.101798
Mikhail E, Pavlovic ZJ, Al Jumaily M, Kheil MH, Moawad GN, Soares T (2022) Robot-assisted surgery for endometriosis current and future perspectives. Surg Technol Int 19(40):197–202. https://doi.org/10.52198/22.STI.40.GY1562
Safiejko K, Tarkowski R, Koselak M, Juchimiuk M, Tarasik A, Pruc M, Smereka J, Szarpak L (2021) Robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection: a systematic review and meta-analysis of 19,731 patients. Cancers 14(1):180. https://doi.org/10.3390/cancers14010180
Guerrini GP, Lauretta A, Belluco C, Olivieri M, Forlin M, Basso S, Breda B, Bertola G, Di Benedetto F (2017) Robotic versus laparoscopic distal pancreatectomy: an up-to-date meta-analysis. BMC Surg 17(1):105. https://doi.org/10.1186/s12893-017-0301-3
Varghese A, Doglioli M, Fader AN (2019) Updates and controversies of robotic-assisted surgery in gynecologic surgery. Clin Obstet Gynecol 62(4):733–748. https://doi.org/10.1097/GRF.0000000000000489
Chandler J, Hopewell S (2013) Cochrane methods–twenty years experience in developing systematic review methods. Syst Rev 20(2):76. https://doi.org/10.1186/2046-4053-2-76
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 22(3):276–282
McGuinness LA, Higgins JPT (2021) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12(1):55–61. https://doi.org/10.1002/jrsm.1411
GRADEpro GDT (2023) GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. Available from www.gradepro.org/
IntHout J, Ioannidis JP, Borm GF (2014) The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 18(14):25. https://doi.org/10.1186/1471-2288-14-25
Harrer M, Cuijpers P, Furukawa T, Ebert D (2021) Doing meta-analysis with R: a hands-on guide. Chapman and Hall/CRC, Boca Raton. https://doi.org/10.1201/9781003107347
Viechtbauer W, Cheung MW (2010) Outlier and influence diagnostics for meta-analysis. Res Synth Methods 1(2):112–125. https://doi.org/10.1002/jrsm.11
IntHout J, Ioannidis JP, Rovers MM, Goeman JJ (2016) Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6(7):e010247. https://doi.org/10.1136/bmjopen-2015-010247
R Core Team (2021) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/
Schwarzer G (2022) Meta: general package for meta-analysis. https://github.com/guido-s/meta/. https://doi.org/10.1007/978-3-319-21416-0
Cuijpers P, Furukawa T, Ebert DD (2021) Dmetar: companion R Package for the guide doing meta-analysis in R. https://dmetar.protectlab.org
Moon HS, Shim JE, Lee SR, Jeong K (2018) The comparison of robotic single-site surgery to single-port laparoendoscopic surgery for the treatment of advanced-stage endometriosis. J Laparoendosc Adv Surg Tech A 28(12):1483–1488. https://doi.org/10.1089/lap.2018.0118
Lee HJ, Lee JS, Lee YS (2020) Comparison of serum antimüllerian hormone levels after robotic-assisted vs. laparoscopic approach for ovarian cystectomy in endometrioma. Eur J Obstet Gynecol Reprod Biol 249:9–13. https://doi.org/10.1016/j.ejogrb.2020.04.010
Raimondo D, Alboni C, Orsini B, Aru AC, Farulla A, Maletta M, Arena A, Del Forno S, Sampogna V, Mastronardi M, Petrillo M, Seracchioli R (2021) Comparison of perioperative outcomes between standard laparoscopic and robot-assisted approach in patients with rectosigmoid endometriosis. Acta Obstet Gynecol Scand 100(9):1740–1746. https://doi.org/10.1111/aogs.14170
Le Gac M, Ferrier C, Touboul C, Owen C, Arfi A, Boudy AS, Jayot A, Bendifallah S, Daraï E (2020) Comparison of robotic versus conventional laparoscopy for the treatment of colorectal endometriosis: pilot study of an expert center. J Gynecol Obstet Hum Reprod. https://doi.org/10.1016/j.jogoh.2020.101885
Nezhat CR, Stevens A, Balassiano E, Soliemannjad R (2015) Robotic-assisted laparoscopy vs conventional laparoscopy for the treatment of advanced stage endometriosis. J Minim Invasive Gynecol 22(1):40–44. https://doi.org/10.1016/j.jmig.2014.06.002
Magrina JF, Espada M, Kho RM, Cetta R, Chang YH, Magtibay PM (2015) Surgical excision of advanced endometriosis: perioperative outcomes and impacting factors. J Minim Invasive Gynecol 22(6):944–950. https://doi.org/10.1016/j.jmig.2015.04.016
Nezhat FR, Sirota I (2014) Perioperative outcomes of robotic assisted laparoscopic surgery versus conventional laparoscopy surgery for advanced-stage endometriosis. JSLS 18(4):e2014.00094. https://doi.org/10.4293/JSLS.2014.00094
Soto E, Luu TH, Liu X, Magrina JF, Wasson MN, Einarsson JI, Cohen SL, Falcone T (2017) Laparoscopy vs. robotic surgery for endometriosis (LAROSE): a multicenter, randomized, controlled trial. Fertil Steril 107(4):996–1002. https://doi.org/10.1016/j.fertnstert.2016.12.033
Chen SH, Li ZA, Du XP (2016) Robot-assisted versus conventional laparoscopic surgery in the treatment of advanced stage endometriosis: a meta-analysis. Clin Exp Obstet Gynecol 43(3):422–426
Restaino S, Mereu L, Finelli A, Spina MR, Marini G, Catena U, Turco LC, Moroni R, Milani M, Cela V, Scambia G, Fanfani F (2020) Robotic surgery vs laparoscopic surgery in patients with diagnosis of endometriosis: a systematic review and meta-analysis. J Robot Surg 14(5):687–694. https://doi.org/10.1007/s11701-020-01061-y
Balla A, Quaresima S, Subiela JD, Shalaby M, Petrella G, Sileri P (2018) Outcomes after rectosigmoid resection for endometriosis: a systematic literature review. Int J Colorectal Dis 33(7):835–847. https://doi.org/10.1007/s00384-018-3082-y. Erratum in: Int J Colorectal Dis. 2018 Jul 9
Feng Z, Feng MP, Feng DP, Solórzano CC (2020) Robotic-assisted adrenalectomy using da Vinci Xi vs Si: are there differences? J Robot Surg 14(2):349–355. https://doi.org/10.1007/s11701-019-00995-2
Hiltunen J, Eloranta ML, Lindgren A, Keski-Nisula L, Anttila M, Sallinen H (2021) Robotic-assisted laparoscopy is a feasible method for resection of deep infiltrating endometriosis, especially in the rectosigmoid area. J Int Med Res 49(8):3000605211032788. https://doi.org/10.1177/03000605211032788
Le Carpentier M, Merlot B, Bot Robin V, Rubod C, Collinet P (2016) Étude comparative : laparoscopie robot assistée versus cœlioscopie chez les patientes avec une endométriose vésicale [Partial cystectomy for bladder endometriosis: Robotic assisted laparoscopy versus standard laparoscopy]. Gynecol Obstet Fertil 44(6):315–321. https://doi.org/10.1016/j.gyobfe.2016.02.006. (French)
Marino P, Houvenaeghel G, Narducci F, Boyer-Chammard A, Ferron G, Uzan C, Bats AS, Mathevet P, Dessogne P, Guyon F, Rouanet P, Jaffre I, Carcopino X, Perez T, Lambaudie E (2015) Cost-effectiveness of conventional vs robotic-assisted laparoscopy in gynecologic oncologic indications. Int J Gynecol Cancer 25(6):1102–1108. https://doi.org/10.1097/IGC.0000000000000458
Lee YJ, Lee DE, Oh HR, Ha HI, Lim MC (2022) Learning curve analysis of multiport robot-assisted hysterectomy. Arch Gynecol Obstet 306(5):1555–1561. https://doi.org/10.1007/s00404-022-06655-5
Terzi H, Biler A, Demirtas O, Guler OT, Peker N, Kale A (2016) Total laparoscopic hysterectomy: analysis of the surgical learning curve in benign conditions. Int J Surg 35:51–57. https://doi.org/10.1016/j.ijsu.2016.09.010
Shugaba A, Lambert JE, Bampouras TM, Nuttall HE, Gaffney CJ, Subar DA (2022) Should all minimal access surgery be robot-assisted? A systematic review into the musculoskeletal and cognitive demands of laparoscopic and robot-assisted laparoscopic surgery. J Gastrointest Surg 26(7):1520–1530. https://doi.org/10.1007/s11605-022-05319-8
Sers R, Forrester S, Zecca M, Ward S, Moss E (2021) The ergonomic impact of patient body mass index on surgeon posture during simulated laparoscopy. Appl Ergon 97:103501. https://doi.org/10.1016/j.apergo.2021.103501
AAGL Advancing Minimally Invasive Gynecology Worldwide (2013) AAGL position statement: robotic-assisted laparoscopic surgery in benign gynecology. J Minim Invasive Gynecol 20(1):2–9. https://doi.org/10.1016/j.jmig.2012.12.007
Sloth SB, Schroll JB, Settnes A, Gimbel H, Rudnicki M, Topsoee MF, Joergensen A, Nortvig H, Moeller C (2017) Systematic review of the limited evidence for different surgical techniques at benign hysterectomy: a clinical guideline initiated by the Danish Health Authority. Eur J Obstet Gynecol Reprod Biol 216:169–177. https://doi.org/10.1016/j.ejogrb.2017.07.012
Ballester M, Roman H (2018) Prise en charge chirurgicale de l’endométriose profonde avec atteinte digestive, RPC Endométriose CNGOF-HAS [Surgical management of deep endometriosis with colorectal involvement: CNGOF-HAS Endometriosis Guidelines]. Gynecol Obstet Fertil Senol. 46(3):290–295. https://doi.org/10.1016/j.gofs.2018.02.003. (French)
Hegyi P, Erőss B, Izbéki F, Párniczky A, Szentesi A (2021) Accelerating the translational medicine cycle: the Academia Europaea pilot. Nat Med 27(8):1317–1319. https://doi.org/10.1038/s41591-021-01458-8
Hegyi P, Petersen OH, Holgate S, Erőss B, Garami A, Szakács Z, Dobszai D, Balaskó M, Kemény L, Peng S, Monteiro J, Varró A, Lamont T, Laurence J, Gray Z, Pickles A, FitzGerald GA, Griffiths CEM, Jassem J, Rusakov DA, Verkhratsky A, Szentesi A (2020) Academia Europaea position paper on translational medicine: the cycle model for translating scientific results into community benefits. J Clin Med 9(5):1532. https://doi.org/10.3390/jcm9051532
Ferrier C, Le Gac M, Kolanska K, Boudy AS, Dabi Y, Touboul C, Bendifallah S, Daraï E (2022) Comparison of robot-assisted and conventional laparoscopy for colorectal surgery for endometriosis: a prospective cohort study. Int J Med Robot. 18(3):e2382. https://doi.org/10.1002/rcs.2382
Dulemba JF, Pelzel C, Hubert HB (2013) Retrospective analysis of robot-assisted versus standard laparoscopy in the treatment of pelvic pain indicative of endometriosis. J Robot Surg 7(2):163–169. https://doi.org/10.1007/s11701-012-0361-4
Nezhat C, Lewis M, Kotikela S, Veeraswamy A, Saadat L, Hajhosseini B, Nezhat C (2010) Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril 94(7):2758–2760. https://doi.org/10.1016/j.fertnstert.2010.04.031
Acknowledgements
None to declare.
Funding
Open access funding provided by Semmelweis University. Sponsors had no role in the design, data collection, analysis, interpretation, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
ÁC: conceptualization, project administration, methodology, formal analysis, data curation, writing—original draft; DPK: project administration, methodology, data curation; ÁJ: project administration, methodology, data curation; AS: conceptualization, writing—review and editing; PF: conceptualization, formal analysis, data curation, visualization, writing—review and editing; ZS: conceptualization, formal analysis, data curation, visualization, writing—review and editing; NÁ: conceptualization; writing—review and editing; PN: conceptualization; writing—review and editing; LS: conceptualization; writing—review and editing; PH: conceptualization, writing—review and editing; IS: conceptualization, writing—review and editing; SV: conceptualization; supervision; writing—review and editing. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Corresponding author
Ethics declarations
Disclosures
Drs. Ádám Csirzó, Dénes Péter Kovács, Anett Szabó, Péter Hegyi, Péter Nyirády, Levente Sára, Nándor Ács, István Szabó, Sándor Valent and Mr. Péter Fehérvári, Árpád Jankó, Zoltán Sipos have no conflicts of interest or financial ties to disclose.
Ethical approval
No ethical approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Csirzó, Á., Kovács, D.P., Szabó, A. et al. Robot-assisted laparoscopy does not have demonstrable advantages over conventional laparoscopy in endometriosis surgery: a systematic review and meta-analysis. Surg Endosc 38, 529–539 (2024). https://doi.org/10.1007/s00464-023-10587-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10587-9