Abstract
Background
To compare the outcomes of blunt splenic injuries (BSI) managed with proximal (P) versus distal (D) versus combined (C) splenic artery embolization (SAE).
Methods
This retrospective study included patients with BSI who demonstrated vascular injuries on angiograms and were managed with SAE between 2001 and 2015. The success rate and major complications (Clavien–Dindo classification ≥ III) were compared between the P, D, and C embolizations.
Results
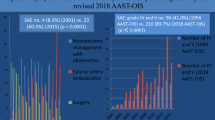
In total, 202 patients were enrolled (P, n = 64, 31.7%; D, n = 84, 41.6%; C, n = 54, 26.7%). The median injury severity score was 25. The median times from injury to SAE were 8.3, 7.0, and 6.6 h for the P, D, and C embolization, respectively. The overall haemostasis success rates were 92.6%, 93.8%, 88.1%, and 98.1% in the P, D, and C embolizations, respectively, with no significant difference (p = 0.079). Additionally, the outcomes were not significantly different between the different types of vascular injuries on angiograms or the materials used in the location of embolization. Splenic abscess occurred in six patients (P, n = 0; D, n = 5; C, n = 1), although it occurred more commonly in those who underwent D embolization with no significant difference (p = 0.092).
Conclusions
The success rate and major complications of SAE were not significantly different regardless of the location of embolization. The different types of vascular injuries on angiograms and agents used in different embolization locations also did not affect the outcomes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
In 1981, Sclafani published the first report of splenic artery embolization (SAE) using gelfoam, steel particles, and vasopressors in three patients with blunt splenic injuries (BSI) as an alternative to surgery [1], and reported a 95.1% spleen salvage rate in 60 patients with BSI using SAE in 1995 [2]. Overtime, the management of BSI has evolved. Nonoperative management (NOM) is the current standard of care for hemodynamically stable patients, and SAE is an integral adjunct to NOM. The splenic artery can be embolized proximally, distally or in combination. However, the location of embolization, its safety, efficacy, and complications remain debatable. For proximal (P) embolization, the goal is to decrease perfusion pressure to achieve hemostasis and the rich network of collateral circulation enters the spleen to decrease the risk of infarction and abscess formation and preserve function [3,4,5,6]. For distal (D) embolization, the goal is to focally devascularize the location of the injury while preserving splenic artery patency, which may lead to wedge infarctions or abscess formation [7, 8]. Combined (C) embolization is defined as the combination of both techniques.
Materials and methods
Study design and period
This study aimed to compare the clinical success and complications in 202 patients with BSI who demonstrated vascular injuries on angiograms and were managed with either P, D, or C embolisation of the splenic artery. Covering a 15-year period from January 1, 2001 to December 31, 2015, data of patients with BSI who underwent angiogram were retrospectively reviewed from the trauma registry and medical records. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital. The requirement for an informed consent was waived owing to the study design, and the personal information of the patients was kept confidential. As an observational cohort study, the STROBE guidelines were observed [9].
Study population
Our hospital is a 3704-bed level 1 trauma center in Northern Taiwan with a multidisciplinary collaboration team, including 24/7 in-house year-round attending trauma surgeons and 24/7 in-house interventional radiologists, and the operating room and angiographic suite were available 24 h. In addition to a multidisciplinary teamwork, clinical guidelines for management of BSI have been established and followed (Fig. 1) [10]. Patients with an Abbreviated Injury Score indicating splenic injury who underwent SAE were included in the study, while those without vascular injuries on angiograms were excluded.
Injury grading and resuscitation
All patients had splenic injuries documented on their admission abdominal computed tomography (CT) scans that were graded and interpreted by trauma surgeons and interventional radiologists. Splenic injuries were graded according to the 2018 American Association for the Surgery of Trauma-Organ Injury Scale (AAST-OIS) revision (Table 1) [11].
Management of protocol
Patients with BSI who are hypotensive and refractory to resuscitation require surgery. All hemodynamically stable patients (including those with shock at triage and response to resuscitation) with grade I or II splenic injuries on admission abdominal CT scans were simply observed in the ward. Patients with grades III, IV, and V without vascular injuries on abdominal CT scans underwent NOM-obs in the intensive-care unit. Patients with vascular injuries on abdominal CT scans were considered for SAE (Fig. 1) [10].
SAE technique
Femoral artery access was obtained by eventual placement of a 4- or 5-French catheter into the celiac trunk. Celiac angiogram was performed to demonstrate not only the splenic artery anatomy but also the collaterals to the spleen, including the dorsal and great pancreatic arteries, and to identify active contrast extravasation, pseudoaneurysm, degree of devitalised spleen, and abnormally truncated vessels. For proximal embolization (Fig. 2), a coil pack is typically placed precisely distal to the main pancreatic artery branches to allow for continued blood supply via the collateral vessels [12, 13], the dorsal pancreatic artery and the pancreatica magna should be identified on angiograms, and embolization is performed between these two branches to avoid devascularization of the pancreas and ischemic pancreatitis. For distal embolization (Fig. 3), the microcatheter is placed as distally as possible in the injured splenic artery branches before embolization to completely preserve the spleen [14]. Combined embolization (Fig. 4) was defined as a combination of both techniques. Various agents, such as coils or absorbable 1–3 mm gelfoam cubes (Upjohn, Inc., Kalamazoo, MI, USA), can be used either alone or in combination. The goal of embolization is to achieve cessation of contrast medium extravasation or total obliteration of the pseudoaneurysm.
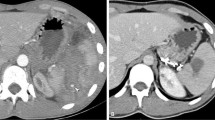
58-Year-old man with a grade IV blunt splenic injury underwent proximal splenic arterial embolization. a Admission contrast-enhanced helical computed tomography scan demonstrates a low attenuation laceration with a big pseudoaneurysm (arrow) and perisplenic hematoma. b The splenic angiogram demonstrates multiple pseudoaneurysms (arrows) at lower half of spleen. c The post-embolization angiogram demonstrates the deployment of metallic coils in the main splenic artery (arrows). Pseudoaneurysm is no longer present
61-Year-old woman with a grade V blunt splenic injury underwent distal splenic arterial embolization. a Admission contrast-enhanced helical computed tomography scan demonstrates splenic laceration with perisplenic contrast medium extravasation (arrows). b The splenic angiogram demonstrates an active leak of contrast medium into the parenchyma with peritoneal cavity extension (arrows) from a branch artery of upper pole. c Metallic coils (arrow) were deployed selectively at upper pole. The post-embolization angiogram demonstrates no further extravasation from the injured spleen
32-Year-old man with a grade IV blunt splenic injury underwent combined splenic arterial embolization. a Admission contrast-enhanced helical computed tomography scan demonstrates a low attenuation laceration with a pseudoaneurysm (arrow) and perisplenic hematoma. b The splenic angiogram demonstrates an active leak of contrast medium from a branch artery of upper pole (arrows). c The post-embolization angiogram demonstrates combined embolization with coils in the proximal splenic artery (arrow) and in the distal branch (arrowheads)
Definition
A pseudoaneurysm was defined as a contrast medium confined within the splenic capsule. Contrast extravasation was defined as the leakage of the injected contrast medium extending beyond the spleen into the peritoneum. Success of the SAE was defined as achieving hemostasis after the first embolization. Spleen salvage was defined as patient discharge with the spleen in situ. Complications were graded as minor or major according to the Clavien–Dindo classification (CDC) [15]. Grade I and II complications that did not require treatment were classified as minor. Grade III, IV, and V complications requiring endoscopic or surgical treatment that were life-threatening or resulted in death were classified as major [15]. In this study, major complications (CDC ≥ III) between the locations of embolization were compared, and asymptomatic splenic infarcts were not included. Spleen-related mortality was defined as death due to persistent bleeding from a splenic injury.
Demographic data analysis
Medical charts were reviewed retrospectively for age, sex, trauma mechanism, injury severity score (ISS), imaging study, location of embolization, and outcomes. Patients were divided into three groups depending on the location of embolization as follows: P, D, and C embolization.
Statistics
Categorical data are presented as numerical values and continuous data as median (i.q.r.) values. The Mann–Whitney U test was used for continuous data. For comparisons of categorical variables, Pearson’s chi-squared test or Fisher's exact test was used, as appropriate. All statistical analyses were performed using the SPSS® version 20.0 (IBM, Armonk, New York, USA). Significance was set at P < 0.05 (two‐sided).
Results
Characteristics of the study population and management
During the study period, 255 patients who underwent angiogram and 53 patients with no vascular injuries with prophylactic embolization (n = 32), no vascular injury without embolization (n = 16), and experienced technical failure (n = 5) were excluded from the study. The remaining 202 patients who demonstrated vascular injuries on angiograms after complete embolization were included in the study (P, n = 64; D, n = 84; C, n = 54) (Fig. 5). Baseline characteristics at admission to the emergency department are presented in Table 2. In total, 166 patients were male, and 36 were female, with a median age of 39.5, 37.5, and 32 years in the P, D, and C embolization groups, respectively (p = 0.544). The median OIS score was 4 for the P, D, and C embolization groups (p = 0.350), and the median ISS was 25 for the P, D, and C embolization groups (p = 0.528). The median time from injury to embolization was 8.3, 7.0, and 6.6 h in the P, D, and C embolization groups, respectively (p = 0.548) (Table 2). No significant differences in sex, age, shock at triage, initial serum hemoglobin level, severity of injuries, length of stay, and time from injury to embolization were observed among the three groups (Table 2). In this series, 187 patients achieved hemostasis after SAE with an overall success rate of 92.6%, and 93.8%, 88.1%, and 98.1% for the P, D, and C embolization groups, respectively, although the difference was not significant (p = 0.079) (Table 3). Pseudoaneurysms were the most common vascular injuries on angiograms in 128 patients (63.4%), and the success rates were 92.8%, 90.9%, and 96.8% for the P, C, and C embolization groups, respectively, which were not significantly different (p = 0.593) (Table 4). The correlation between the success rate of other vascular injuries and embolization location is presented in Table 4. Coils or microcoils alone were the most commonly used embolizers in 167 patients (82.7%), and the success rates were 94.0%, 87.9%, and 98.0% for P, D, and C embolizations, respectively, and were not significantly different (p = 0.112) (Table 5). Table 5 presents the success rates of the other embolizers according to the location of embolization. Major complications (CDC III) such as post-embolization splenic abscess that required further intervention occurred in six patients, with no significant difference among the three groups (p = 0.092) (Table 3). Five patients died after SAE, with an overall mortality rate of 2.5%, and four of them died of head cause; the other patient died of splenic rebleeding despite receiving emergent surgery, with a spleen-related mortality rate of 0.5% (Table 3).
Discussion
Currently, a higher success rate of SAE of 90% has been reported [2, 10, 14, 16]. The splenic artery can be embolized proximally, distally or in combination. P embolization is the most common choice and is preferred in some reports [2, 6, 12, 14, 17], D embolization in others [18,19,20], and C embolization in some series [7, 8, 14, 17, 21, 22]. P embolization has a shorter procedural time and presents with a quicker option for providing treatment in multiple splenic injuries (Fig. 2), even with significant anatomic variation and/or vasospasm [14, 16, 23]. However, P embolization can lead to permanent occlusion of the splenic artery and precludes further angiographic management if rebleeding occurs. Compared to P embolization, D embolization is more technically challenging, and it is beneficial in patients with focal, angiographically evident injury and in minimizing global splenic ischemia (Fig. 3). However, segmental bleeding vessels may be overlooked because of vasospasms caused by haematomas, potentially increasing the risk of rebleeding [4, 8, 14, 18]. Combined embolization is reserved for vascular lesions combined with high-grade splenic injuries, and for some vascular injuries, it may not be visible on the initial angiogram and may lead to delayed bleeding once the vasospasm subsides [5, 21, 22, 24]. Reports over the past few years have mainly discussed conflicting clinical outcomes of P versus D embolization, and the optimal location of embolization remains inconclusive [5, 8, 12, 14, 16, 17]. Reports simultaneously comparing the outcomes of P, D, and C embolizations are scant [21, 22]. Our data are the largest series of SAE collected to date from a single center, and we graded our patients using the 2018 AAST-OIS which incorporates vascular injury that facilitates recognition of grade IV and V injuries and is superior to the 1994 classification [10]. Moreover, patients who demonstrated no vascular injuries on angiograms and underwent prophylactic embolization were excluded to minimize bias as much as possible and to demonstrate the outcomes of different locations of embolization. In this report, we analyze and discuss two parts: first, the comparison of P, D, and C embolizations in terms of safety and efficacy of hemostasis and, second, the major complications requiring intervention (CDC ≥ III) of these three embolizations. A meta-analysis of 876 patients from 1991 to 2013 by Rong et al. [16] has revealed the following: P embolization was performed more often than distal embolization and combined significantly (52.1% vs. 24.8% vs. 5.5%, respectively); the overall success rate of SAE was 90.1% (range 72.7–100%); and success rate and embolization location (P > 0.05) had no evident association. Conversely, distal embolization was the most common choice in our series (n = 84, 41.6%), followed by P embolization (n = 64, 31.7%) and combined embolization (n = 54, 26.7%) (Table 2). Fifteen patients (P, n = 4; D, n = 10; C, n = 1) with persistent bleeding in our series failed to undergo SAE; the success rate was 92.6% overall, and 93.8%, 88.1%, and 98.1% in the P, D, and C embolization groups, respectively; however, the differences were not significant (p = 0.079) (Table 3). One patient who underwent P embolization initially and experienced rebleeding with sudden hemodynamic collapse at the ward 9 days later and died despite receiving emergent surgery. However, no significant difference in mortality was observed between the embolization locations (p = 0.584) (Table 3). Of the 15 patients who had failed initial SAE with persistent bleeding, nine (P, n = 3; D, n = 5; C, n = 1) underwent splenectomy, and the other six (P, n = 1; D, n = 5) achieved hemostasis after a repeat embolization with an overall salvage rate of 95.5%, and of 95.3%, 94.0%, and 98.1% in the P, D, and C embolizations, respectively; the differences were not significant (p = 0.519) (Table 3). These data confirm that no significant difference exists between the success rate and the location of embolization, which is in accordance with a previous review [16]. In addition to comparing the success according to the location of embolization, we compared the efficacy of hemostasis in different vascular injuries on angiograms with the location of embolization, which has not been previously reported. In this series, pseudoaneurysm was the most common vascular injury demonstrated on angiograms (n = 128, 63.4%), followed by combined contrast extravasation with pseudoaneurysm (n = 36, 17.8%) and contrast extravasation (n = 35, 17.3%) (Table 4). Based on our data, despite the various types of vascular injuries on angiograms, the success rate was not significantly different according to the location of embolization (Table 4). Coil, the most commonly used material for embolization, can be deposited more accurately, although it may cause migration when undersized to the vessel and rebleeding or more infraction of the spleen [8, 14, 18]. Meanwhile, gelfoam is a water-insoluble, nonelastic hemostatic agent that slows blood flow, hastens thrombus formation, and provides temporary vessel occlusion, allowing recanalization within days or a few weeks [4, 25]. At present, the correlation between the embolization material and severe complications remains controversial. Using resorbable agents reportedly have a higher risk of rebleeding and a higher severe complication rate than using coil [16, 17, 21]. However, other studies have suggested that the difference was not significant [14, 19]. In our study, 167 patients (82.7%) underwent coil embolization, 12 patients (5.9%) underwent gelfoam embolization, and 23 patients (11.4%) underwent combined coil and gelfoam embolization (Table 5). Our data revealed that the success rate of embolization was independent of the embolic material and location of embolization (Table 5). Except for rebleeding, other complications of SAE include splenic infarct, cyst, abscess, and contrast-induced renal insufficiency [8, 16]. In a study by Ekeh et al. [8], the significant complication rate remained higher in individuals who underwent distal embolization than in those who underwent P embolization (22% vs. 4%; p = 0.02). Splenic infarction is the most common post-embolization complication; however, the vast majority of these patients are asymptomatic and can be managed nonoperatively [14, 16]. In our protocol, an abdominal CT scan was not routinely performed after SAE unless indicated in selected cases; therefore, data on asymptomatic splenic infarcts are incomplete. In Rong et al.’s review [16], although P embolization had fewer CDC III complications than distal embolization (9.9% vs. 20.0%) or combined embolization (10.8% vs. 23.1%), the p value were 0.07 (P vs. D) and 0.16 (P vs. C), respectively, with no significant differences. Reportedly, splenic abscess formation after SAE occurs in 3.8–7% of patients [8, 16]. In our series, splenic abscess after SAE occurred in six patients (3.0%); five of these underwent CT-guided drainage, and the other underwent surgical drainage. Although splenic abscess occurred more commonly in the distal embolization group (n = 5), no significant difference was observed among the three groups (p = 0.092) (Table 3).
This study had some limitations. First, as this was a retrospective study, the subsequent availability of data is limited to those that were present in the patient’s medical records. Prospective studies are needed to determine the optimal location of embolization for the management of BSI. Second, as this was a single-center study, the internal structure of the trauma and interventional radiology teams may not necessarily reflect that of other hospitals.
Conclusions
The clinical success rate of SAE was 92.6%, and the splenic salvage rate was 95.5%, which confirmed that SAE for BSI was associated with good success rates. Irrespective of P, D, or C embolization, SAE are effective for hemorrhage control. There was also no statistically significant difference between the location of embolization and major complications.
The different types of vascular injuries on angiograms and embolic agents used also did not impact the outcomes, regardless of the location of embolization.
References
Sclafani SJ (1981) The role of angiographic hemostasis in salvage of the injured spleen. Radiology 141:645–650
Sclafani SJ, Shaftan GW, Scalea TM, Patterson LA, Kohl LDO, Kantor A, Herskowitz MM, Hoffer EK, Henry S, Dresner LS, Wetzel W (1995) Non-operative salvage of computed tomography-diagnosed splenic injuries: utilization of angiography for triage and embolization for hemostasis. J Trauma 39:818–827
Pirasteh A, Snyder LL, Lin R, Rosenblum D, Reed S, Sattar A, Passalacqua M, Prologo JD (2012) Temporal assessment of splenic function in patients who have undergone percutaneous image-guided splenic artery embolization in the setting of trauma. J Vasc Interv Radiol 23:80–82
Imbrogno BF, Ray CE (2012) Splenic artery embolization in blunt trauma. Semin Interv Radiol 29:147–149
Requarth JA (2010) Distal splenic artery hemodynamic changes during transient proximal splenic artery occlusion in blunt splenic injury patients: a mechanism of delayed splenic hemorrhage. J Trauma 69:1423–1426
Bessoud B, Denys A, Calmes JM, Madoff D, Qanadli S, Schnyder P (2006) Nonoperative management of traumatic splenic injuries: is there a role for proximal splenic artery embolization? Am J Roentgenol 186:779–785
Killeen KL, Shanmuganathan K, Boyd-Kranis R, Scalea TM, Mirvis SE (2001) CT findings after embolization for blunt splenic trauma. J Vasc Interv Radiol 12:209–214
Ekeh AP, Khalaf S, Ilyas S, Kauffman S, Walusimbi M, McCarthy MC (2013) Complications arising from splenic artery embolization: a review of an 11-year experience. Am J Surg 205:250–254
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) STROBE initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Lin BC, Wu CH, Wong YC, Chen HW, Fu CJ, Huang CC, Wu CT, Hshieh CH (2023) Splenic artery embolization changes the management of blunt splenic injury: an observational analysis of 680 patients graded by the revised 2018 AAST-OIS. Surg Endosc 37:371–381. https://doi.org/10.1007/s00464-022-09531-0
Kozar RA, Crandall M, Shanmuganathan K, Zarzaur BL, Coburn M, Cribari C, Kaups K, Schuster K, Tominaga GT (2018) Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg 85:1119–1122
Sabe AA, Claridge JA, Rosenblum DI, Lie K, Malangoni MA (2009) The effects of splenic artery embolization on nonoperative management of blunt splenic injury: a 16-year experience. J Trauma 67:565–572
van der Vlies CH, Hoekstra J, Ponsen KJ, Reekers JA, van Delden OM, Goslings JC (2012) Impact of splenic artery embolization on the success rate of nonoperative management for blunt splenic injury. Cardiovasc Intervent Radiol 35:76–81
Haan JM, Biffl W, Knudson MM, Davis KA, Oka T, Majercik S, Dicker R, Marder S, Scalea TM (2004) Splenic embolization revisited: a multicenter review. J Trauma 56:542–547
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Rong JJ, Liu D, Liang M, Wang QH, Sun JY, Zhang QY, Peng CF, Xuan FQ, Zhao LJ, Tian XX, Hanet YL (2017) The impacts of different embolization techniques on splenic artery embolization for blunt splenic injury: a systematic review and meta-analysis. Mil Med Res 4:17
Smith HE, Biffl WL, Majercik SD, Jednacz J, Lambiase R, Cioffi WG (2006) Splenic artery embolization: Have we gone too far? J Trauma 61:541–544
Dasgupta N, Matsumoto AH, Arslan B, Turba UC, Sabri S, Angle JF (2012) Embolization therapy for traumatic splenic lacerations. Cardiovasc Intervent Radiol 35:795–806
Wu SC, Chen RJ, Yang AD, Tung CC, Lee KH (2008) Complications associated with embolization in the treatment of blunt splenic injury. World J Surg 32:476–482
Wei B, Hemmila MR, Arbabi S, Taheri PA, Wahl WL (2008) Angioembolization reduces operative intervention for blunt splenic injury. J Trauma 64:1472–1477
Frandon J, Rodière M, Arvieux C, Michoud M, Vendrell A, Broux C, Sengel C, Bricault I, Ferretti G, Thony F (2014) Blunt splenic injury: outcomes of proximal versus distal and combined splenic artery embolization. Diagn Interv Imaging 95:825–831
Entriken C, Weed Z, Parikh PP, Ekeh AP (2022) Complications following splenic embolization for trauma: have things changed over time? J Surg Res 277:44–49
Requarth JA, Miller PR (2012) The splenic artery stump pressure is affected by arterial anatomy after proximal embolotherapy in blunt splenic injury. J Trauma Acute Care Surg 73:1221–1224
Haan JM, Marmery H, Shanmuganathan K, Mirvis SE, Scalea TM (2007) Experience with splenic main coil embolization and significance of new or persistent pseudoaneurym: reembolize, operate, or observe. J Trauma 63:615–619
Abada HT, Golzarian J (2007) Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol 10:257–260
Acknowledgements
We are grateful to Miss Shu-Fang Huang for her statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Drs. Being-Chuan Lin, Cheng-Hsien Wu, Yon-Cheong Wong, Huan-Wu Chen, Chen-Ju Fu, Chen-Chih Huang, Chen-Te Wu, Yi-Kang Ku, Chien-Cheng Chen, Ting-Wen Sheng, and Chun-Bi, Chang have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, BC., Wu, CH., Wong, YC. et al. Comparison of outcomes of proximal versus distal and combined splenic artery embolization in the management of blunt splenic injury: a report of 202 cases from a single trauma center. Surg Endosc 37, 4689–4697 (2023). https://doi.org/10.1007/s00464-023-09960-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-09960-5