Abstract
Background
Submucosal tunneling endoscopic resection (STER) and endoscopic submucosal excavation (ESE) are less-invasive therapeutic alternatives to surgical resection for the removal of esophageal or gastric submucosal tumors (SMTs). This study aimed to comparing STER versus ESE for the resection of esophageal and gastric SMTs from the muscularis propria.
Methods
This systematic review and meta-analysis was reported in accordance with PRISMA guidelines through December 2020. Pooled outcome measures included complete resection, en bloc resection, bleeding, perforation, adverse events, recurrence, procedure duration, and length of hospital stay. Risk ratio (RR) and mean difference (MD) was calculated as well as Peto time-to-event analyses to determine recurrence rate.
Results
Five retrospective cohort studies (n = 269 STER versus n = 319 ESE) were included. There was no difference in rates of complete resection [RR: 1.01 (95% CI 0.94, 1.07)], en bloc resection [RR: 0.95 (95% CI 0.84, 1.08)], recurrence [OR: 1.18 (95% CI 0.33, 4.16)], and total adverse events [RR: 1.33 (95% CI 0.78, 2.27)]. Specific adverse events including rates of perforation [RR: 0.57 (95% CI 0.12, 2.74)] and bleeding [RR: 1.21 (95% CI 0.30, 4.88)] were not different between STER and ESE. There was a statistical difference when evaluating procedure time, with the STER group presenting significantly larger values [MD: 24.62 min (95% CI 20.04, 29.20)].
Conclusion
STER and ESE were associated with similar efficacy and safety; however, ESE was associated with a significantly decreased time to complete the procedure.
Similar content being viewed by others
Submucosal tumors (SMTs), also known as subepithelial lesions (SELs) or subepithelial tumors (SETs), may be present throughout the entire gastrointestinal tract [1]. These types of lesions are commonly found during routine endoscopic investigation [2], most commonly identified as incidental findings [1, 3]. Submucosal tumors may appear as protrusions in the organ wall, with mucosa unaltered and equivalent to adjacent ones. The vast majority of these patients are generally asymptomatic, though in in a minority of cases, these lesions may cause pain, obstruction, or gastrointestinal hemorrhage [3, 4]. It remains pivotal to perform endoscopic ultrasonography (EUS) to more accurately evaluate these lesions and identify SMT topography and echogenicity, aiding the etiological diagnosis of these lesions [1, 5, 6].
The differential for SELs remains highly variable, depending on the layer of the organ wall from which the tumor has emerged [3, 7]. Endoscopy alone is insufficient for accurate diagnosis [6]. Among the potential etiologies include gastrointestinal stromal tumors (GISTs), which present malignant potential and should be resected if causing symptoms or larger than 2 cm, according to National Comprehensive Cancer Network guidelines [8]. Other potential etiologies include leiomyoma, lipoma, pancreatic rest, granular cell tumor, carcinoid, inflammatory gastric polyp, gastric varix, schwannoma, lymphangioma, or duplication cyst.
For years, surgical resection was the only therapeutic option for SMTs arising from the muscularis propria layer, due to its intimate contact with the serous membrane [9]. Conventional endoscopic resection techniques, such as endoscopic submucosal dissection (ESD), presented a considerable risk of perforations [10] and risk of incomplete resection for cases involving lesions larger than 2 cm [11, 12]. Aiming to provide a safe approach with impressive resection results, minimally invasive techniques such as submucosal tunneling endoscopic resection (STER) and endoscopic submucosal excavation (ESE) were developed [13].
Given the introduction of STER and ESE as available endoscopic resection techniques, we aimed to perform a structured systematic review and meta-analysis to compare the efficacy and safety of these modalities for resection of SMTs arising from the muscularis propria.
Materials and methods
Protocol and registration
This systematic review and meta-analysis was reported in accordance with the 'Preferred Reporting Items for Systematic Reviews and Meta-analyses' (PRISMA) [14]. The study was submitted to the Prospective Register of Systematic Reviews (PROSPERO) and registered under number CRD42020185511. This study was approved by the Ethics-Scientific Committee of the Department of Gastroenterology at the University of Sao Paulo Medical School. Due to the study format, informed consent was not necessary.
Eligibility criteria and information sources
The initial search was conducted considering published studies with no restrictions concerning language or publication year. The selected studies followed the following criteria: (1) participants: patients with submucosal tumors from the muscularis propria at the esophagus or stomach; (2) intervention: submucosal tunneling endoscopic resection; (3) comparison: endoscopic submucosal excavation; and (4) results: complete resection, en bloc resection, recurrence, adverse events, perforation, bleeding, procedure time, and hospital stay.
Exclusion criteria were the following: (1) studies with non-human subjects; (2) studies including subjects younger than 18 years; (3) studies that assessed the analyzed techniques only in other parts of the gastrointestinal tract; and (4) studies that did not discriminate outcomes according to each intervention group.
Search strategy and study selection
Searches were conducted electronically using on-line databases (MEDLINE, EMBASE and Cochrane Library), through December 31, 2020. The same search strategy was applied for all previous databases, namely: ((esophagus OR gastric OR stomach) AND (endoscopic mucosal resection OR submucosal tunneling resection techniques OR submucosal tunnel dissection OR microscopy scanning tunneling OR STER OR tunneling endoscopic muscularis dissection OR EFTR OR endoscopic full thickness resection OR endoscopic submucosal excavation)). The studies were selected based on the aforementioned exclusion and eligibility criteria. Two independent researchers conducted the screening following the eligibility conditions. Divergences were settled in consensus or after consulting a third reviewer.
Data collection process
After study selection, two reviewers extracted the required information and formatted the data into tables. Primary outcomes were complete resection (R0), en bloc resection, total adverse events, and local recurrence. Secondary outcomes stratified adverse events such as bleeding and perforation as well as procedure time, and hospital stay. Due to the intrinsic risk of perforation as a result of resections of lesions at the muscularis propria, this outcome was defined as a discontinuity in the organ wall that required surgical interventions beyond endoscopy.
Risk of bias in individual studies
In order to assess the risk of bias in cohort studies, we employed the Risk of Bias in Non-randomized Studies (ROBINS-I) [15] (Table 1). This tool is structured into seven bias domains: (1) confounding; (2) selection of participants; (3) classification of interventions; (4) deviations from intended interventions; (5) missing data; (6) measurement of outcomes; (7) selection of the reported result. We analyzed the individual risk of bias for each included study.
Statistical analysis and quality of evidence
The analysis was conducted using the Review Manager software, version 5.4 (RevMan 5.4; Cochrane Collaboration, Oxford, UK). Risk ratio (RR) for dichotomous variables was calculated using a fixed-effects model. To appropriately determine rate of recurrence, a time-to-event analysis was utilized using the Peto Odds ratio according the Cochrane Handbook working methodology. For continuous variables, expressed in absolute values, the differences between measures were calculated using the mean difference (MD)—employing the mean, the standard deviation, and the size of the sample for each group). In case of studies that failed to report mean and variance values, those were estimated from the median, the interval and the size of the sample [16]. We adopted a confidence interval of 95% and the level of statistical significance was established as a p-value lower than 0.05. To assess heterogeneity, we conducted heterogeneity level (I2) analyses to identify publication bias and the percentage of variation across studies. We considered I2 greater than 50% as high heterogeneity. Quality of evidence was assessed according to the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) (Table 2).
Results
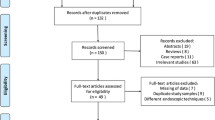
A total of 3528 articles were identified, 3219 through our searches on MEDLINE via PubMed and other 309 found on EMBASE and Cochrane Library databases. Duplicates were removed. After applying the eligibility criteria, 7 studies of retrospective cohorts in full text were chosen; however, only 5 of which remained and were included in the meta-analysis [17,18,19,20,21]. The two excluded studies failed to present their data classified by intervention and comparison groups [22, 23]. The selection process is presented in Fig. 1 and Table 3 describes the individual characteristics of the selected studies. All 5 of the included retrospective cohort studies [17,18,19,20,21] specified their inclusion and exclusion criteria, including and comparing 269 patients in the intervention group (STER) and 319 in the control group (ESE). One of the cohort studies presented additional data concerning endoscopic full thickness resection (EFTR) [17] that was not analyzed.
Risk of bias
All studies were assessed by ROBINS-I [15], with 3 studies classified as moderate risk [17, 19, 21] and 2 as serious risk [18, 20]. The detailed risk of bias for each included study is described in the complementary material (Table 1).
Individual results from studies
Complete resection (R 0)
All 5 studies [17,18,19,20,21] included in this meta-analysis reported the outcome of complete resection. A total of 588 patients were evaluated, of them 269 were in the STER group and 319 in the ESE group. There was no significant statistical difference between groups [RR: 1.01 (95% CI 0.94, 1.07); I2 = 58%; p = 0.87] (Fig. 2). A low quality of evidence was defined (Table 2).
En bloc resection
Three studies [17, 19, 21] documented en bloc resection. In total, 411 patients were evaluated, with 180 in the STER group and 231 in the ESE group. There was no significant statistical difference between groups [RR: 0.95 (95% CI 0.84, 1.08); I2 = 51%; p = 0.41] (Fig. 3). A moderate quality of evidence was identified (Table 2).
Recurrence
All 5 selected studies [17,18,19,20,21] evaluated rates of local recurrence. A total of 588 patients were assessed, of these 269 were in the STER group and 319 in the ESE group. According to the analysis (Fig. 4), there was no significant statistical difference between the two groups [OR: 1.18 (95% CI 0.33, 4.16); I2 = 0%; p = 0.80] and a very low quality of evidence was found (Table 2).
Adverse events
All 5 studies [17,18,19,20,21] presented data concerning adverse events. In a preliminary analysis, we verified the presence of high heterogeneity (I2 = 64%). In total, 588 patients were evaluated, of them 269 were in the STER group and 319 in the ESE group. There was no significant statistical difference between groups [RR: 1.33 (95% CI 0.78, 2.27); I2 = 64%; p = 0.30] (Fig. 5). We next identified the presence of outlier study [20]. After removal of this outlier study, heterogeneity levels became very low (I2 = 0%). Afterward ,494 patients were evaluated, of which 225 were in the STER group and 269 in the ESE group, finding no significant statistical difference between groups [RR: 1.17 (95% CI 0.93, 1.47); I2 = 0%; p = 0.19] (Fig. 6). Therefore, 4 studies [17,18,19, 21] were included for this outcome. Quality of evidence was defined as moderate (Table 2).
Perforation
All 5 studies [17,18,19,20,21] presented data regarding perforation for this meta-analysis. A total of 588 patients were evaluated (n = 269 in the STER group and n = 319 in the ESE group). There was no significant statistical difference between groups [RR: 0.57 (95% CI 0.12, 2.74); I2 = 72%; p = 0.49] (Fig. 7). There was a very low quality of evidence defined (Table 2).
Bleeding
All 5 studies [17,18,19,20,21] reported post-resection hemorrhage outcomes. In total, 588 patients were assessed, with n = 269 in the STER group vs n = 319 in the ESE group. There was no significant statistical difference between groups [RR: 1.21 (95% CI 0.30, 4.88); I2 = 15%; p = 0.79] (Fig. 8) and a low quality of evidence was found (Table 2).
Procedure time
Four studies [18,19,20,21] were considered in this outcome. In total, 431 patients were assessed, with n = 226 in the STER group and n = 205 in the ESE group. There was a statistical difference between groups, with significantly higher values in the STER group, which presented a mean difference of 24.62 min longer in comparison with ESE [MD: 24.62 min (95% CI 20.04, 29.20); I2 = 38%; p < 0.00001] (Fig. 9). The quality of evidence for this outcome was defined as low (Table 2).
Length of hospital stay
Three studies [17, 19, 21] were included in this outcome. In total, 411 patients were evaluated, with 180 in the STER group and 231 in the ESE group. There was no significant statistical difference between groups [MD: 0.61 (95% CI − 0.23, 1.45); I2 = 95%; p = 0.15] (Fig. 10) and a very low quality of evidence was defined (Table 2).
Discussion
Although STER and ESE are considered safe and viable techniques [24] for the resection of submucosal tumors from the muscularis propria layer, it is not possible to define a technical superior procedure at this time due to the absence of robust data and incongruity of published data. In this systematic review and meta-analysis, we evaluated submucosal tunneling endoscopic resection (STER) and endoscopic submucosal excavation (ESE) techniques, currently the most widespread methods for the resection of esophageal and gastric SMTs originating from the muscularis propria. Both compared resection techniques appeared to be effective and safe; however, ESE was associated with a shorter procedure time. There was no difference in rates of complete resection, en bloc resection, recurrence, and total adverse events including rates of bleeding and perforation.
In 2012, Xu et al. first described the STER technique [25]. The technique involved a 2 cm longitudinal incision approximately 5 cm from the lesion, followed by the creation of a submucous tunnel until the exposure and removal of the lesion was complete [25]. The STER technique is similar to per-oral endoscopic myotomy (POEM) as utilized for the endoscopic treatment of achalasia. The SEL is then resected using a snare or a knife while in the submucosal tunnel and retrieved prior to closure of the defect using endoscopic clips or suture. Closure then improves post-resection healing, which decreases the risk of adverse events such as subcutaneous emphysema and pneumomediastinum [25, 26]. In 2017, Xiu-He et al. published a systematic review assessing SMT resection in the upper gastrointestinal tract by means of the STER technique, demonstrating that STER is a viable and safe option for treating these types of tumors [26].
In contrast to STER, the endoscopic submucosal excavation (ESE) technique is performed via a longitudinal or circular incision in the mucosa above the lesion, followed by the dissection of the layers until the tumor is found. Once this is accomplished and the lesion removed, the provider attempts to approximate the edges of the remaining mucosa with metal endoscopic clips [27]. ESE is derived and perfected from an endoscopic submucous dissection (ESD) technique [10], which is a more widespread resection technique, but not suitable for lesions arising from deeper layers. Previous literature has shown ESE technique to be safe and effective for both esophageal [24] and gastric lesions [28, 29] at the muscularis propria.
When evaluating rates of complete resection (R0) and en bloc resection we found no significant statistical difference between the patients that underwent STER vs ESE. Despite the groups being similar in many aspects, it should be noted that these groups were not homogenous throughout the included studies, with variation in regard to tumor topography and lesion size (Table 4). It remains important to acknowledge this may have influenced the results within these outcomes due to the anatomical variation at the resection site. In the included study, Xiu et al. [17] conducted an analysis utilizing subgroups categorized according to the location of the resected lesions, and showed that both STER and ESE presented no significant difference regarding complete and en bloc resection of esophageal lesions. An analysis concerning only gastric lesions was not possible due to the low number of SMTs resected by means of STER.
In a subgroup analysis, Xu et al. [20] demonstrated that tumors ≤ 15 mm had a complete resection rate with both ESE and STER of 100%. For tumors > 15 mm, the STER group had a higher rate of complete resection as well as adverse events compared to the ESE group. Xiu et al. [17] showed there is no significant difference between the rates of complete and en bloc resections of lesions < 20 mm and lesions between 20 and 40 mm.
Two previous systematic reviews, which considered only the STER technique, demonstrated complete (R0) and en bloc resection rates of 97% and 94%, respectively [26, 30]. Another prospective study assessing only the ESE technique showed complete and en bloc resection rates of 95% and 96%, respectively [31]. These previous literature is in accordance with the results found in this present systematic review and meta-analysis. Individually, the studies suggest that lesions smaller than 10 mm to 15 mm may be safely resected by both techniques and that larger lesions may be preferably resected by STER, despite lower rates of en bloc resection of lesions of 4 cm or larger [17, 18, 20, 21].
With regard to rates of recurrence, there was no statistical difference between the techniques. However, it is important to acknowledge that the follow-up period varied among studies and the histologic etiology of each lesion was only defined in a post-procedure assessment of the resected tissue (Table 4). The most commonly found tumors were leiomyomas followed by GISTs, which are associated with higher recurrence risk [3]. Among the included studies, the study by Xu et al. [20] presented the largest sample of GIST lesions. Still, no patient in this study developed local recurrence after endoscopic resection, reinforcing the safety of both techniques, even among lesions with traditionally higher rates of recurrence. As our systematic review and meta-analysis has shown, other studies confirm that both STER [26, 30, 32,33,34,35,36,37,38,39] and ESE [28, 31] present low recurrence rates.
The assessment of perforation rate in our study also demonstrated no significant statistical difference between STER and ESE. The absence of a uniformly-established definition for all studies regarding this outcome, and the lack of information regarding the use of carbon dioxide (CO2) flow applied in the procedures, compromise its level of evidence. It is important to highlight that the ESE technique presents alternative variations regarding the incision performed in the mucosa, namely the longitudinal [31] and circumferential [27] incisions. Among the studies selected in our analysis, 2 presented circumferential incisions [17, 19] while 3 utilized a longitudinal incision [18, 20, 21]. The latter allowed an easier approximation of the mucosa with metal clips at the end of the procedure, which has been associated with a decreased risk of a clinically significant perforation. Furthermore, our study showed no significant statistical difference concerning bleeding rate between the groups. Post-procedure hemorrhage was associated with a low incidence for both techniques, occurring in only 3 cases among all patients submitted to STER (3/269) and 3 events among patients undergoing ESE (3/319).
As for the safety of the techniques, Lu et al. [18] performed subgroup analyses among lesions of different sizes, and showed that lesions < 10 mm reached 100% of complete resection rate, and there was no perforation or adverse symptoms related to the procedure. For lesions > 10 mm, despite the high rate of complete resection (ESE: 92%, STER: 97%), the perforation rate was also high (ESE: 16%, STER: 18.2%). Both STER and ESE had a similar perforation rate (3%, p < 0.05).
Not surprisingly, the length of hospital stay was also similar—both being endoscopic treatments with reduced length of stay compared to traditional surgical resection. Importantly, however, ESE was associated with a shorter procedure time compared to STER. The STER technique may demand a longer execution time possibly because it requires the formation of a tunnel by dissection until the lesion is exposed [40]. This occurs in contrast to ESE, which dissects the organ wall layers at a place equivalent to the lesion site. Despite all studies being retrospective, and there being a need for more high quality data, we believe that this meta-analysis provides important information that may help in decision-making. We acknowledge that the average size of the lesions removed by the STER technique is slightly higher in all studies included in the meta-analysis, and thus, may be a form of selection bias that makes the time to perform the procedure longer.
When evaluating the individual studies, respective authors attempted to reduce this bias. Du et al. reported in their study that no difference was found between the groups when assessing the size of the lesions (p < 0.05). The study by Lu et al. performed a detailed assessment by subgroups, confirming that to resect lesions > 10 mm, performing STER requires more time. In an analysis of subgroups, Xu et al. confirmed that the mean time of operation was longer with STER than with ESE for tumors ≤ 15 mm and that both groups had a similar time for lesions > 15 mm. Contrary to the other meta-analyzed studies, Chen et al. showed that tumors ≥ 20 mm have similar operating time for both STER and ESE (p > 0.05) and that for tumors < 20 mm, STER was performed in a shorter time (p < 0.05).
It is important to acknowledge this systematic review and meta-analysis is not without other limitations. Chiefly, this study included only observational studies—which is not surprising given the novelty of the endoscopic techniques. Despite this, it is possible the decision to perform one technique over the other may result in significant selection bias and inability to control for unmeasured confounders. Additionally, given the complexity of these procedure, it is likely these results will not translate to a broader clinical practice, and will continue to be performed by a small group of endoscopists in a few centers with expertise. As such, the well-designed randomized clinical trials or prospective studies among these institutions as well as registry databases are needed. Furthermore, adoption of a rigorous definition concerning the criteria for lesion selection, measured outcome assessment, and implementation are required.
Conclusion
Overall, STER and ESE are both considered safe and effective techniques for the resection of esophageal and gastric SMTs arising from the muscularis propria. The choice between STER and ESE should thus follow the particularities of each institution, prioritizing the technique for which the endoscopist is more skilled and trained, as well as availability of resources. Ultimately, the ESE technique was associated with a shorter procedure time compared to STER. However, there was no significant difference between the techniques with regard to rates of complete resection (R0), en bloc resection, adverse events, local recurrence, and length of hospital stay.
References
Kim GH (2012) Endoscopic resection of subepithelial tumors. Clin Endosc 45:240. https://doi.org/10.5946/ce.2012.45.3.240
Bang CS, Baik GH, Shin IS, Suk KT, Yoon JH, Kim DJ (2016) Endoscopic submucosal dissection of gastric subepithelial tumors: a systematic review and meta-analysis. Korean J Intern Med 31:860–871. https://doi.org/10.3904/kjim.2015.093
Humphris JL, Jones DB (2008) Subepithelial mass lesions in the upper gastrointestinal tract. J Gastroenterol Hepatol 23:556–566. https://doi.org/10.1111/j.1440-1746.2007.05232.x
Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T (2008) Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 13:416–430. https://doi.org/10.1007/s10147-008-0798-7
Nickl N (2005) Endoscopic approach to gastrointestinal stromal tumors. Gastrointest Endosc Clin N Am 15:455–466. https://doi.org/10.1016/j.giec.2005.04.001
McCarty TR, Ryou M (2020) Endoscopic diagnosis and management of gastric subepithelial lesions. Curr Opin Gastroenterol 36:530–537. https://doi.org/10.1097/MOG.0000000000000674
Ponsaing LG (2007) Classification of submucosal tumors in the gastrointestinal tract. World J Gastroenterol 13:3311. https://doi.org/10.3748/wjg.v13.i24.3311
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PWT, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD (2010) NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 8(Suppl 2):S1–S41 (quiz S42–4)
Ponsaing LG (2007) Therapeutic procedures for submucosal tumors in the gastrointestinal tract. World J Gastroenterol 13:3316. https://doi.org/10.3748/wjg.v13.i24.3316
Kim SY, Kim K-O (2018) Endoscopic treatment of subepithelial tumors. Clin Endosc 51:19–27. https://doi.org/10.5946/ce.2018.020
Biaek A, Wiechowska-Kozowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpiska K, Awniczak M, Starzyska T (2012) Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc 75:276–286. https://doi.org/10.1016/j.gie.2011.08.029
Chun SY, Kim KO, Park DS, Lee IJ, Park JW, Moon S-H, Baek IH, Kim JH, Park CK, Kwon MJ (2013) Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc 27:3271–3279. https://doi.org/10.1007/s00464-013-2904-9
Zhang H, Huang X, Qu C, Bian C, Xue H (2019) Comparison between laparoscopic and endoscopic resections for gastric submucosal tumors. Saudi J Gastroenterol 25:245. https://doi.org/10.4103/sjg.SJG_412_18
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1. https://doi.org/10.1186/2046-4053-4-1
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. https://doi.org/10.1136/bmj.i4919
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Xiu H, Zhao C-Y, Liu F-G, Sun X-G, Sun H, Liu X-S (2019) Comparing about three types of endoscopic therapy methods for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Scand J Gastroenterol 54:1481–1486. https://doi.org/10.1080/00365521.2019.1692064
Lu J, Jiao T, Zheng M, Lu X (2014) Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc 28:3401–3407. https://doi.org/10.1007/s00464-014-3610-y
Du C, Chai N, Linghu E, Gao Y, Li Z, Li L, Zhai Y, Lu Z, Meng J, Tang P (2018) Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation. Surg Endosc 32:4543–4551. https://doi.org/10.1007/s00464-018-6206-0
Xu H-W, Zhao Q, Yu S-X, Jiang Y, Hao J-H, Li B (2019) Comparison of different endoscopic resection techniques for submucosal tumors originating from muscularis propria at the esophagogastric junction. BMC Gastroenterol 19:174. https://doi.org/10.1186/s12876-019-1099-5
Chen Y, Wang M, Zhao L, Chen H, Liu L, Wang X, Fan Z (2020) The retrospective comparison between submucosal tunneling endoscopic resection and endoscopic submucosal excavation for managing esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc 34:417–428. https://doi.org/10.1007/s00464-019-06785-z
Zhang Y, Mao X-L, Zhou X-B, Yang H, Chen L-HZG, Ye L-P (2018) Long-term outcomes of endoscopic resection for small (≤ 4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer. World J Gastroenterol 24:3030–3037. https://doi.org/10.3748/wjg.v24.i27.3030
Guo Y, Jing X, Zhang J, Ding X, Li X, Mao T, Tian Z (2019) Endoscopic removal of gastrointestinal stromal tumors in the stomach: a single-center experience. Gastroenterol Res Pract 2019:1–9. https://doi.org/10.1155/2019/3087298
Reinehr R (2015) Die Endoskopische Submukosaexkavation (ESE) ist für ausgesuchte Fälle eine sichere und praktikable Technik zur endoskopischen Entfernung submuköser Tumoren von Magen und Speiseröhre. Z Gastroenterol 53:573–578. https://doi.org/10.1055/s-0034-1399384
Xu M-D, Cai M-Y, Zhou P-H, Qin X-Y, Zhong Y-S, Chen W-F, Hu J-W, Zhang Y-Q, Ma L-L, Qin W-Z, Yao L-Q (2012) Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 75:195–199. https://doi.org/10.1016/j.gie.2011.08.018
Lv X-H, Wang C-H, Xie Y (2017) Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc 31:49–63. https://doi.org/10.1007/s00464-016-4978-7
Zhang Y (2015) Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol 21:9503. https://doi.org/10.3748/wjg.v21.i32.9503
Wang S, Shen L (2016) Efficacy of endoscopic submucosal excavation for gastrointestinal stromal tumors in the cardia. Surg Laparosc Endosc Percutan Tech 26:493–496. https://doi.org/10.1097/SLE.0000000000000330
Wang Y, Li Y, Luo H, Yu H (2014) Efficacy analysis of endoscopic submucosal excavation for gastric gastrointestinal stromal tumors. Zhonghua Wei Chang Wai Ke Za Zhi 17:352–355
Jain D (2017) Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol 30:262–272. https://doi.org/10.20524/aog.2017.0128
Zhang Y, Ye L, Zhu L, Zhou X, Mao X, Ding J (2013) Endoscopic muscularis excavation for subepithelial tumors of the esophagogastric junction originating from the muscularis propria layer. Dig Dis Sci 58:1335–1340. https://doi.org/10.1007/s10620-012-2487-7
Chen T, Zhou P-H, Chu Y, Zhang Y-Q, Chen W-F, Ji Y, Yao L-Q, Xu M-D (2017) Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg 265:363–369. https://doi.org/10.1097/SLA.0000000000001650
Zhou D-J (2015) Submucosal tunneling and endoscopic resection of submucosal tumors at the esophagogastric junction. World J Gastroenterol 21:578. https://doi.org/10.3748/wjg.v21.i2.578
Wang X-Y, Xu M-D, Yao L-Q, Zhou P-H, Pleskow D, Li Q-L, Zhang Y-Q, Chen W-F, Zhong Y-S (2014) Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc 28:1971–1977. https://doi.org/10.1007/s00464-014-3420-2
Du C, Chai N-L, Ling-Hu E-Q, Li Z-J, Li L-S, Zou J-L, Jiang L, Lu Z-S, Meng J-Y, Tang P (2019) Submucosal tunneling endoscopic resection: an effective and safe therapy for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. World J Gastroenterol 25:245–257. https://doi.org/10.3748/wjg.v25.i2.245
Ye L-P, Zhang Y, Mao X-L, Zhu L-H, Zhou X, Chen J-Y (2014) Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 28:524–530. https://doi.org/10.1007/s00464-013-3197-8
Li Z, Gao Y, Chai N, Xiong Y, Ma L, Zhang W, Du C, Linghu E (2018) Effect of submucosal tunneling endoscopic resection for submucosal tumors at esophagogastric junction and risk factors for failure of en bloc resection. Surg Endosc 32:1326–1335. https://doi.org/10.1007/s00464-017-5810-8
Li Q-L, Chen W-F, Zhang C, Hu J-W, Zhou P-H, Zhang Y-Q, Zhong Y-S, Yao L-Q, Xu M-D (2015) Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc 29:3640–3646. https://doi.org/10.1007/s00464-015-4120-2
Wang H, Tan Y, Zhou Y, Wang Y, Li C, Zhou J, Duan T, Zhang J, Liu D (2015) Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol 27:776–780. https://doi.org/10.1097/MEG.0000000000000394
Xu M, Zhang C, Hu J, Chen T, Zhou P, Zhong Y, Zhang Y, Chen W, Li Q, Yao L (2014) Submucosal tunneling endoscopic resection for upper gastrointestinal multiple submucosal tumors originating from the muscular propria layer: a feasibility study. Indian J Cancer 51:52. https://doi.org/10.4103/0019-509X.151989
Funding
None.
Author information
Authors and Affiliations
Contributions
FLPN: acquisition of data, analysis, interpretation of data, drafting the article, revising the article, final approval; DTHM: analysis and interpretation of data, revising the article; FCM: analysis and interpretation of data, revising the article; VMTS: acquisition of data, analysis, interpretation of data, drafting the article, revising the article, final approval; IBR: analysis and interpretation of data, revising the article; MBB: analysis and interpretation of data, revising the article; TRM: analysis and interpretation of data, revising the article; NTM: analysis and interpretation of data, revising the article; Ide, E:, revising the article and english; WMB: analysis and interpretation of data, drafting the article, final approval; EGHM analysis and interpretation of data, drafting the article, revising the article, final approval.
Corresponding author
Ethics declarations
Disclosures
Fernando Lopes Ponte Neto, Diogo Turiani Hourneaux de Moura, Vitor Massaro Takamatsu Sagae, Igor Braga Ribeiro, Fabio Catache Mancini, Mateus Bond Boghossian, Thomas R. McCarty, Nelson Tomio Miyajima, Edson Ide, and Wanderley Marques Bernardo declare that they have no conflict of interest. Dr. Eduardo Guimarães Hourneaux de Moura reports personal fees from Boston Scientific and Olympus but these were not relevant to this manuscript.
Ethical approval
The study was approved by the Research Ethics Committee of the University of São Paulo School of Medicine Hospital das Clínicas.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ponte Neto, F.L., de Moura, D.T.H., Sagae, V.M.T. et al. Endoscopic resection of esophageal and gastric submucosal tumors from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation: A systematic review and meta-analysis. Surg Endosc 35, 6413–6426 (2021). https://doi.org/10.1007/s00464-021-08659-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08659-9