Abstract
Background
Angiopoetin- (Ang-) 1 inhibits and Ang-2 promotes VEGF-mediated angiogenesis via binding to endothelial cell-bound Tie-2 receptor (Tie-2). After minimally invasive colorectal resection (MICR), Ang-1 levels decrease and Ang-2 levels increase, which may stimulate angiogenesis in wounds and residual tumor foci. Soluble Tie-2 (sTie-2) modulates the effects of free Ang-1 and Ang-2 by binding to them. This study assessed perioperative MICR plasma sTie-2 levels.
Methods
Blood samples were taken preoperatively (PreOp) and on postoperative days (POD) 1 and 3 from 50 cancer and 53 benign disease MICR patients. In a subgroup, a fourth sample was taken between POD7 and POD13 and bundled as a single time point. sTie-2 levels (ng/ml) were determined via ELISA. The mean and SD were determined at each time point. The t test used for analysis.
Results
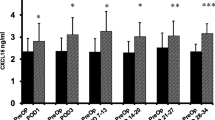
PreOp plasma sTie-2 levels were significantly higher in the benign group (27.6 ± 10.2) than in the cancer group (22.9 ± 7.9). A significant drop from PreOp occurred in sTie-2 levels in the cancer group on POD1 (20.0 ± 7.4) and POD3 (21.0 ± 6.6) and in the benign group on POD1 (24.8 ± 9.1). The benign group’s POD3 and the cancer group’s POD7-13 sTie-2 levels were statistically similar to the PreOp levels while the benign group’s POD7-13 level was significantly higher.
Conclusion
PreOp sTie-2 levels were significantly lower in cancer patients. MICR is associated with a significant short-lived decrease in plasma sTie-2 levels in cancer patients on POD1 and 3, which may briefly inhibit VEGF-mediated angiogenesis. The benign group’s early results were similar.


Similar content being viewed by others
References
Leather AJ, Gallegos NC, Kocjan G, Savage F, Smales CS, Hu W, Boulos PB, Northover JM, Phillips RK (1993) Detection and enumeration of circulating tumour cells in colorectal cancer. Br J Surg 80(6):777–780
Kirman I, Cekic V, Poltaratskaia N, Asi Z, Bessler M, Huang EH, Forde KA, Whelan RL (2002) Plasma from patients undergoing major open surgery stimulates in vitro tumor growth: lower insulin-like growth factor binding protein 3 levels may, in part, account for this change. Surgery 132(2):186–192
Kirman I, Poltoratskaia N, Sylla P, Whelan RL (2004) Insulin-like growth factor-binding protein 3 inhibits growth of experimental colocarcinoma. Surgery 136(2):205–209
Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL (1998) Increased tumor establishment and growth after open vs. laparoscopic bowel resection in mice. Surg Endosc 12(8):1035–1038
Carter JJ, Feingold DL, Kirman I, Oh A, Wildbrett P, Asi Z, Fowler R, Huang E, Whelan RL (2003) Laparoscopic-assisted cecectomy is associated with decreased formation of postoperative pulmonary metastases compared with open cecectomy in a murine model. Surgery 134(3):432–436
Weese JL, Ottery FD, Emoto SE (1986) Do operations facilitate tumour growth? An experimental model in rats. Surgery 100(2):273–277
Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, Proud G, Taylor RM (1985) Influence of surgical operations on components of the human immune system. Br J Surg 72(10):771–776
Eggermont AM, Steller EP, Sugerbaker PH (1987) Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery 102(1):71–78
Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL (1999) Increased tumor establishment and growth after open versus laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc 13(3):233–235
Folkman J, Cole P, Zimmerman S (1966) Tumor behavior in isolated perfused organs: In vitro growth and metastasis of biopsy material in rabbit thyroid and canine intestinal segment. Ann Surg 164(3):491–502
Belizon A, Balik E, Horst P, Feingold D, Arnell T, Azarani T, Cekic V, Skitt R, Kumara S, Whelan RL (2008) Persistent elevation of plasma VEGF levels during the first month following minimally invasive colorectal resection. Surg Endosc 22(2):287–297
Shantha Kumara HMC, Feingold D, Kalady M, Dujovny N, Senagore A, Hyman N, Cekic V, Whelan RL (2009) Colorectal resection is associated with persistent proangiogenic plasma protein changes: postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann Surg 249(6):973–977
Reusch P, Barleon B, Weindel K, Martiny-Baron G, Gödde A, Siemeister G, Marmé D (2001) Identification of a soluble form of the angiopoietin receptor TIE-2 released from endothelial cells and present in human blood. Angiogenesis 4(2):123–131
Findley CM, Cudmore MJ, Ahmed A, Kontos CD (2007) VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol 27(12):2619–2626
Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG (2005) The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 118:771–780
Yuan HT, Khankin EV, Karumanchi SA, Parikh SM (2009) Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in endothelium. Mol Cell Biol 29(8):2011–2022
Maisonpierre PC, Suri C, Jones PF (1997) Angiopoietin-2a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277(5322):55–60
Nadar SK, Blann A, Beevers DG, Lip GY (2005) Abnormal angiopoietins 1 & 2, angiopoietin receptor Tie-2 and vascular endothelial growth factor levels in hypertension: relationship to target organ damage. J Intern Med 258(4):336–343
Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY (2004) Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor Tie-2 levels in congestive heart failure. J Am Coll Cardiol 43(3):423–428
Chung NA, Makin AJ, Lip GY (2003) Measurement of the soluble angiopoietin receptor Tie-2 in patients with coronary artery disease: development and application of an immunoassay. Eur J Clin Invest 33(7):529–535
Lee KW, Lip GY, Blann AD (2004) Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor Tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation 110(16):2355–2360
Koutroubakis IE, Xidakis C, Karmiris K, Sfiridaki A, Kandidaki E, Kouroumalis EA (2006) Potential role of soluble angiopoietin-2 and Tie-2 in patients with inflammatory bowel disease. Eur J Clin Invest 36(2):127–132
Kaipainen A, Vlaykova T, Hatva E, Böhling T, Jekunen A, Pyrhönen S, Alitalo K (1994) Enhanced expression of the Tie receptor tyrosine kinase messenger RNA in the vascular endothelium of metastatic melanomas. Cancer Res 54(24):6571–6577
Salvén P, Joensuu H, Heikkilä P, Matikainen MT, Wasenius VM, Alanko A, Alitalo K (1996) Endothelial Tie growth factor receptor provides antigenic marker for assessment of breast cancer angiogenesis. Br J Cancer 74(1):69–72
Nakayama T, Hatachi G, Wen CY, Yoshizaki A, Yamazumi K, Niino D, Sekine I (2005) Expression and significance of Tie-1 and Tie-2 receptors, and angiopoietins-1, 2 and 4 in colorectal adenocarcinoma: Immunohistochemical analysis and correlation with clinicopathological factors. World J Gastroenterol 11(7):964–969
Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K (1997) Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest 100(8):2072–2078
Chin KF, Greenman J, Reusch P, Gardiner E, Marme D, Monson J (2004) Changes in serum soluble VEGFR-1 and Tie-2 receptors in colorectal cancer patients following surgical resections. Anticancer Res 24(4):2353–2357
Belizon A, Balik E, Feingold DL, Bessler M, Arnell TD, Forde KA, Horst PK, Jain S, Cekic V, Kirman I, Whelan RL (2006) Major abdominal surgery increases plasma levels of vascular endothelial growth factor: open more so than minimally invasive methods. Ann Surg 244(5):792–798
Kirman I, Belizon A, Balik E, Feingold D, Arnell T, Horst P, Kumara S, Cekic V, Jain S, Nasar A, Whelan RL (2007) Perioperative sargramostim (recombinant human GM-CSF) induces an increase in the level of soluble VEGFR1 in colon cancer patients undergoing minimally invasive surgery. Eur J Surg Oncol 33(10):1169–1176
Mels AK, Statius Muller MG, van Leeuwen PA, von Blomberg BM, Scheper RJ, Cuesta MA, Beelen RH, Meijer S (2001) Immune-stimulating effects of low-dose perioperative recombinant granulocyte-macrophage colony-stimulating factor in patients operated on for primary colorectal carcinoma. Br J Surg 88(4):539–544
Shantha Kumara HMC, Kirman I, Feingold D, Cekic V, Nasar A, Arnell T, Balik E, Hoffman A, Baxter R, Conte S, Whelan RL (2009) Perioperative GMCSF limits the proangiogenic plasma protein changes associated with colorectal cancer resection. Eur J Surg Oncol 35(3):295–301
Zaman K, Driscoll R, Hahn D, Werffeli P, Goodman SL, Bauer J, Leyvraz S, Lejeune F, Stupp R, Rüegg C (2006) Monitoring multiple angiogenesis-related molecules in the blood of cancer patients shows a correlation between VEGF-A and MMP-9 levels before treatment and divergent changes after surgical vs. conservative therapy. Int J Cancer 118(3):755–764
Disclosures
H. M. C. Shantha Kumara, Michael J. Grieco, Xiaohong Yan, Matthew F. Kalady, Vincent DiMaggio, Donald G Kim, Neil Hyman, Daniel L. Feingold, and Richard L. Whelan have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shantha Kumara, H.M.C., Grieco, M.J., Yan, X. et al. Minimally invasive colorectal resection for cancer is associated with a short-lived decrease in soluble Tie-2 receptor levels, which may transiently inhibit VEGF-mediated angiogenesis (via altered blood levels of free Ang-1 and Ang-2). Surg Endosc 24, 2581–2587 (2010). https://doi.org/10.1007/s00464-010-1008-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1008-z