Abstract
Background
Laparoscopic surgery preserves the immune system and has anti-inflammatory properties. CO2 pneumoperitoneum attenuates lipopolysaccharide (LPS)-induced cytokine production and increases survival. We tested the hypothesis that CO2 pneumoperitoneum mediates its immunomodulatory properties via stimulation of the cholinergic pathway.
Methods
In the first experiment, rats (n = 68) received atropine 1 mg/kg or saline injection 10 min prior to LPS injection and were randomization into four 30-min treatment subgroups: LPS only control, anesthesia control, CO2 pneumoperitoneum, and helium pneumoperitoneum. In a second experiment, rats (n = 40) received atropine 2 mg/kg or saline 10 min prior to randomization into the same four subgroups described previously. In a third experiment, rats (n = 96) received atropine 2 mg/kg or saline 10 min prior to randomization into eight 30-min treatment subgroups followed by LPS injection: LPS only control; anesthesia control; and CO2 or helium pneumoperitoneum at 4, 8, and 12 mmHg. In a fourth experiment, rats (n = 58) were subjected to bilateral subdiaphragmatic truncal vagotomy or sham operation. Two weeks postoperatively, animals were randomized into four 30-min treatment subgroups followed by LPS injection: LPS only control, anesthesia control, CO2 pneumoperitoneum, and helium pneumoperitoneum. Blood samples were collected from all animals 1.5 h after LPS injection, and cytokine levels were determined by enzyme-linked immunosorbent assay.
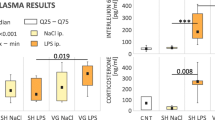
Results
Serum tumor necrosis factor-α (TNF-α) levels were consistently suppressed among the saline–CO2 pneumoperitoneum groups compared to saline–LPS only control groups (p < 0.05 for all four experiments). All chemically vagotomized animals had significantly reduced TNF-α levels compared to their saline-treated counterparts (p < 0.05 for all), except among the CO2 pneumoperitoneum-treated animals. Increasing insufflation pressure with helium eliminated differences (p < 0.05) in TNF-α production between saline- and atropine-treated groups but had no effect among CO2 pneumoperitoneum-treated animals. Finally, vagotomy (whether chemical or surgical) independently decreased LPS-stimulated TNF-α production in all four experiments.
Conclusion
CO2 pneumoperitoneum modulates the immune system independent of the vagus nerve and the cholinergic pathway.





Similar content being viewed by others
References
Are C, Hardacre JM, Talamini MA, Murata K, Frank S (2003) Decreased cardiac output in humans during laparoscopic antireflux surgery: direct measurements. J Laparoendosc Adv Surg Tech A 13: 139–146
Are C, Talamini MA, Murata K, De Maio A (2002) Carbon dioxide pneumoperitoneum alters acute-phase response induced by lipopolysaccharide. Surg Endosc 16: 1464–1467
Bachman SL, Hanly EJ, Nwanko JI, Lamb J, Herring AE, Marohn MR, De Maio A, Talamini MA (2004) The effect of timing of pneumoperitoneum on the inflammatory response. Surg Endosc 18: 1640–1644
Barkun JS, Wexler MJ, Hinchey EJ, Thibeault D, Meakins JL (1995) Laparoscopic versus open inguinal herniorrhaphy: preliminary results of a randomized controlled trial. Surgery 118: 703–710
Berguer R, Cornelius T, Dalton M (1997) The optimum pneumoperitoneum pressure for laparoscopic surgery in the rat mode: a detailed cardiorespiratory study. Surg Endosc 11: 915–918
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462
Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND (2004) Stress response to laparoscopic surgery. Surg Endosc 18: 1022–1028
Dahn S, Schwalbach P, Wohleke F, Benner A, Kuntz C (2003) Influence of different gases used for laparoscopy (helium, carbon dioxide, room air, xenon) on tumor volume, proliferation, and apoptosis. Surg Endosc 17: 1653–1657
Dalton M, Hildreth J, Matsuoka T, Berguer R (1996) Determination of cardiorespiratory function and the optimum anesthetic regimen during laparoscopic surgery in the rat model. Surg Endosc 10: 297–300
Fitzgerald SD, Andrus CH, Baudendistel LJ, Dahms TE, Kaminski DL (1992) Hypercarbia during carbon dioxide pneumoperitoneum. Am J Surg 163: 186–190
Fuentes JM, Hanly EJ, Bachman SL, Aurora AR, Marohn MR, Talamini MA (2004) Videoendoscopic endotracheal intubation in the rat: a comprehensive rodent model of laparoscopic surgery. J Surg Res 122: 240–248
Hanly EJ, Bachman SL, Marohn MR, Boden JH, Herring AE, De Maio A, Talamini MA (2005) CO2-pneumoperitoneum-mediated attenuation of the inflammatory response is independent of systemic acidosis. Surgery 137: 559–566
Hanly EJ, Fuentes JM, Aurora AR, Bachman SL, Marohn MR, De Maio A, Talamini MA (2005) CO2 pneumoperitoneum prevents mortality from sepsis. Surg Endosc 19: S176
Hanly EJ, Mendoza-Sagaon M, Murata K, Hardacre JM, De Maio A, Talamini MA (2003) CO2 pneumoperitoneum modifies the inflammatory response to sepsis. Ann Surg 237: 343–350
Hardacre JM, Talamini MA (2000) Pulmonary and hemodynamic changes during laparoscopy: are they important? Surgery 127: 241–244
Jatzko GR, Lisborg PH, Pertl AM, Stettner HM (1995) Multivariate comparison of complications after laparoscopic cholecystectomy and open cholecystectomy. Ann Surg 221: 381–386
Mann C, Boccara G, Grevy V, Navarro F, Fabre JM, Colson P (1997) Argon pneumoperitoneum is more dangerous than CO2 pneumoperitoneum during venous gas embolism. Anesth Analg 85: 1367–1371
Neuhaus SJ, Gupta A, Watson DI (2001) Helium and other alternative insufflation gases for laparoscopy. Surg Endosc 15: 553–560
Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N, Szekely M (1997) Febrile responsiveness of vagotomized rats is suppressed even in the absence of malnutrition. Am J Physiol 273: 777–783
Rudston-Brown B, Draper PN, Warriner B, Walley KR, Phang PT (1997) Venous gas embolism: a comparison of carbon dioxide and helium in pigs. Can J Anaesth 44: 1102–1107
Schietroma M, Carlei F, Franchi L, Mazzotta C, Sozio A, Lygidakis NJ, Amicucci G (2004) A comparison of serum interleukin-6 concentrations in patients treated by cholecystectomy via laparotomy or laparoscopy. Hepatogastroenterology 51: 1595–1599
Sietses C, von Blomberg ME, Eijsbouts QA, Beelen RH, Berends FJ, Cuesta MA (2002) The influence of CO2 versus helium insufflation or the abdominal wall lifting technique on the systemic immune response. Surg Endosc 16: 525–528
Tracey KJ (2002) The inflammatory reflex. Nature 420: 853–859
Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF (1995) Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett 183: 27–31
Acknowledgments
This work was supported by National Institutes of Health grant R01-GM062899-02. The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paper presented at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), Fort Lauderdale, Florida, USA, April 2005
Rights and permissions
About this article
Cite this article
Fuentes, J.M., Hanly, E.J., Aurora, A.R. et al. Laparoscopic surgery and the parasympathetic nervous system. Surg Endosc 20, 1225–1232 (2006). https://doi.org/10.1007/s00464-005-0280-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-0280-9