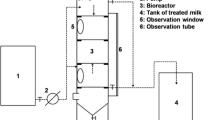
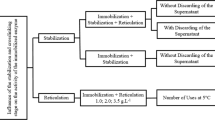
Abstract
The objective of this study was to develop a bioprocess for lactose hydrolysis in diverse dairy matrices, specifically skim milk and cheese whey, utilizing column reactors employing a core–shell enzymatic system featuring β-galactosidase fused to a Cellulose Binding Domain (CBD) tag (β-galactosidase-CBD). The effectiveness of reactor configurations, including ball columns and toothed columns operating in packed and fluidized-bed modes, was evaluated for catalyzing lactose hydrolysis in both skim milk and cheese whey. In a closed system, these reactors achieved lactose hydrolysis rates of approximately 50% within 5 h under all evaluated conditions. Considering the scale of the bioprocess, the developed enzymatic system was capable of continuously hydrolyzing 9.6 L of skim milk while maintaining relative hydrolysis levels of approximately 50%. The biocatalyst, created by immobilizing β-galactosidase-CBD on magnetic core-shell capsules, exhibited exceptional operational stability, and the proposed bioprocess employing these column reactors showcases the potential for scalability.





Similar content being viewed by others
Data availability
Data supporting this study are included within the manuscript.
References
Rueda N, Albuquerque TL, Bartolome-Cabrero R et al (2016) Reversible immobilization of lipases on heterofunctional octyl-amino agarose beads prevents enzyme desorption. Molecules 21:646. https://doi.org/10.3390/MOLECULES21050646
Vera C, Guerrero C, Aburto C et al (2020) Conventional and non-conventional applications of β-galactosidases. Biochim Biophys Acta Proteins Proteom 1868:140271. https://doi.org/10.1016/J.BBAPAP.2019.140271
Bellé AS, Hackenhaar CR, Spolidoro LS et al (2018) Efficient enzyme-assisted extraction of genipin from genipap (Genipa americana L.) and its application as a crosslinker for chitosan gels. Food Chem 246:266–274. https://doi.org/10.1016/J.FOODCHEM.2017.11.028
Balthazar CF, Pimentel TC, Ferrão LL et al (2017) Sheep milk: physicochemical characteristics and relevance for functional food development. Compr Rev Food Sci Food Saf 16:247–262. https://doi.org/10.1111/1541-4337.12250
Duan F, Sun T, Zhang J et al (2022) Recent innovations in immobilization of β-galactosidases for industrial and therapeutic applications. Biotechnol Adv 61:108053. https://doi.org/10.1016/J.BIOTECHADV.2022.108053
Shafi A, Husain Q (2022) Immobilization of β-galactosidase on concanavalin a modified silica-coated titanium dioxide nanocomposite and its interaction with monovalent and divalent cations. Mater Today Commun 32:103828. https://doi.org/10.1016/J.MTCOMM.2022.103828
Lima PC, Gazoni I, de Carvalho AMG et al (2021) β-galactosidase from Kluyveromyces lactis in genipin-activated chitosan: an investigation on immobilization, stability, and application in diluted UHT milk. Food Chem 349:129050. https://doi.org/10.1016/J.FOODCHEM.2021.129050
Argenta AB, Nogueira A, de Scheer PA (2021) Hydrolysis of whey lactose: Kluyveromyces lactis β-galactosidase immobilisation and integrated process hydrolysis-ultrafiltration. Int Dairy J 117:105007. https://doi.org/10.1016/J.IDAIRYJ.2021.105007
Hackenhaar CR, Rosa CF, Flores EEE et al (2022) Development of a biocomposite based on alginate/gelatin crosslinked with genipin for β-galactosidase immobilization: performance and characteristics. Carbohydr Polym 291:119483. https://doi.org/10.1016/J.CARBPOL.2022.119483
Estevinho BN, Samaniego N, Talens-Perales D et al (2018) Development of enzymatically-active bacterial cellulose membranes through stable immobilization of an engineered β-galactosidase. Int J Biol Macromol 115:476–482. https://doi.org/10.1016/J.IJBIOMAC.2018.04.081
Tavares GF, Xavier MR, Neri DFM, de Oliveira HP (2016) Fe3O4@polypyrrole core-shell composites applied as nanoenvironment for galacto-oligosaccharides production. J Chem Eng 306:816–825. https://doi.org/10.1016/J.CEJ.2016.08.018
Zhao W, Yang RJ, Qian TT et al (2013) Preparation of novel Poly(hydroxyethyl methacrylate-co-glycidyl methacrylate)-grafted core-shell magnetic chitosan microspheres and immobilization of lactase. Int J Mol Sci 14:12073–12089. https://doi.org/10.3390/IJMS140612073
Wu Z, Wang Z, Guan B et al (2013) Improving the properties of β-galactosidase from Aspergillus oryzae via encapsulation in aggregated silica nanoparticles. New J Chem 37:3793–3797. https://doi.org/10.1039/C3NJ00685A
Ishiguro T, Obata A, Nagata K et al (2022) Core–shell fibremats comprising a poly(AM/DAAM)/ADH nanofibre core and nylon6 shell layer are an attractive immobilization platform for constructing immobilised enzymes. RSC Adv 12:34931–34940. https://doi.org/10.1039/D2RA06620C
Galogahi FM, Zhu Y, An H, Nguyen NT (2020) Core-shell microparticles: generation approaches and applications. J Sci-Adv Mater Dev 5:417–435. https://doi.org/10.1016/J.JSAMD.2020.09.001
Kurian M, Thankachan S (2021) Structural diversity and applications of spinel ferrite core - shell nanostructures- a review. Open Ceramics 8:100179. https://doi.org/10.1016/J.OCERAM.2021.100179
Zhou Y, Li Y, Hou Y et al (2023) Core-shell catalysts for the elimination of organic contaminants in aqueous solution: a review. Chem Eng J 455:140604. https://doi.org/10.1016/J.CEJ.2022.140604
Bolivar JM, López-Gallego F (2020) Characterization and evaluation of immobilized enzymes for applications in flow reactors. Curr Opin Green Sustain Chem 25:100349. https://doi.org/10.1016/J.COGSC.2020.04.010
Kowalczykiewicz D, Szymańska K, Gillner D, Jarzębski AB (2021) Rotating bed reactor packed with heterofunctional structured silica-supported lipase. Developing an effective system for the organic solvent and aqueous phase reactions. Micropor Mesopor Mat 312:110789. https://doi.org/10.1016/J.MICROMESO.2020.110789
Hama S, Tamalampudi S, Yoshida A et al (2011) Enzymatic packed-bed reactor integrated with glycerol-separating system for solvent-free production of biodiesel fuel. Biochem Eng J 55:66–71. https://doi.org/10.1016/J.BEJ.2011.03.008
da Natividade SJ, Matte CR, Charqueiro DS et al (2017) Directed immobilization of CGTase: The effect of the enzyme orientation on the enzyme activity and its use in packed-bed reactor for continuous production of cyclodextrins. Process Biochem 58:120–127. https://doi.org/10.1016/J.PROCBIO.2017.04.041
Van Zessen E, Tramper J, Rinzema A, Beeftink HH (2005) Fluidized-bed and packed-bed characteristics of gel beads. Chem Eng J 115:103–111. https://doi.org/10.1016/J.CEJ.2005.08.016
Costa-Silva TA, Carvalho AKF, Souza CRF et al (2021) Enhancement lipase activity via immobilization onto chitosan beads used as seed particles during fluidized bed drying: application in butyl butyrate production. Appl Catal A Gen 622:118217. https://doi.org/10.1016/J.APCATA.2021.118217
Bianchi P, Williams JD, Kappe CO (2020) Oscillatory flow reactors for synthetic chemistry applications. J Flow Chem 10:475–490. https://doi.org/10.1007/S41981-020-00105-6/FIGURES/17
Tafete GA, Habtu NG (2023) Reactor configuration, operations and structural catalyst design in process intensification of catalytic reactors: a review. Chem Eng Process 184:109290. https://doi.org/10.1016/J.CEP.2023.109290
Gennari A, Simon R, de Andrade BC et al (2021) Production of beta-galactosidase fused to a cellulose-binding domain for application in sustainable industrial processes. Bioresour Technol 326:124747. https://doi.org/10.1016/J.BIORTECH.2021.124747
Gennari A, Mobayed FH, Da Rolt NB et al (2019) Immobilization of β-galactosidases on magnetic nanocellulose: textural, morphological, magnetic, and catalytic properties. Biomacromol 20:2315–2326. https://doi.org/10.1021/ACS.BIOMAC.9B00285
Gennari A, Simon R, de Moura Sperotto ND et al (2022) One-step purification of a recombinant beta-galactosidase using magnetic cellulose as a support: rapid immobilization and high thermal stability. Bioresour Technol 345:126497. https://doi.org/10.1016/J.BIORTECH.2021.126497
Rech R, Cassini CF, Secchi A, Ayub MAZ (1999) Utilization of protein-hydrolyzed cheese whey for production of β-galactosidase by Kluyveromyces marxianus. J Ind Microbiol Biotechnol 23:91–96. https://doi.org/10.1038/SJ.JIM.2900692
Shafi A, Ahmed F, Husain Q (2021) β-Galactosidase mediated synthesized nanosupport for the immobilization of same enzyme: Its stability and application in the hydrolysis of lactose. Int J Biol Macromol 184:57–67. https://doi.org/10.1016/J.IJBIOMAC.2021.06.034
Mateo C, Palomo JM, Fernandez-Lorente G et al (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463. https://doi.org/10.1016/J.ENZMICTEC.2007.01.018
Gennari A, Simon R, de Moura Sperotto ND et al (2022) Application of cellulosic materials as supports for single-step purification and immobilization of a recombinant β-galactosidase via cellulose-binding domain. Int J Biol Macromol 199:307–317. https://doi.org/10.1016/J.IJBIOMAC.2022.01.006
Pender K, Yang L (2019) Investigation of catalyzed thermal recycling for glass fiber-reinforced epoxy using fluidized bed process. Polym Compos 40:3510–3519. https://doi.org/10.1002/PC.25213
Lorenzoni ASG, Aydos LF, Klein MP et al (2015) Continuous production of fructooligosaccharides and invert sugar by chitosan immobilized enzymes: comparison between in fluidized and packed bed reactors. J Mol Catal B Enzym 111:51–55. https://doi.org/10.1016/J.MOLCATB.2014.11.002
Hussain A, Minh LQ, Lee M (2017) Intensification of the ethylbenzene production process using a column configured with a side reactor. Chem Eng Process 122:204–212. https://doi.org/10.1016/J.CEP.2017.10.003
Gilbert EM, Agrawal S, Schwartz T et al (2015) Comparing different reactor configurations for partial nitritation/anammox at low temperatures. Water Res 81:92–100. https://doi.org/10.1016/J.WATRES.2015.05.022
Dal Magro L, Pessoa JPS, Klein MP et al (2021) Enzymatic clarification of orange juice in continuous bed reactors: fluidized-bed versus packed-bed reactor. Catal Today 362:184–191. https://doi.org/10.1016/J.CATTOD.2020.02.003
Uytdenhouwen Y, Meynen V, Cool P, Bogaerts A (2020) The potential use of core-shell structured spheres in a packed-bed DBD plasma reactor for CO2 conversion. Catalysts 10:530. https://doi.org/10.3390/CATAL10050530
Szab E, Szakos D, Kasza G, Ózsvari L (2021) Analysis of the target group of lactose-free functional foods for product development. Acta Aliment 50:153–161. https://doi.org/10.1556/066.2020.00168
Sainz-García SH, López GL, Alvarado VM et al (2022) Adaptive control for narrow bandwidth input and output disturbance rejection for a non-isothermal CSTR system. Mathematics 10:1–29
Shu S, Vidal D, Bertrand F, Chaouki J (2019) Multiscale multiphase phenomena in bubble column reactors: a review. Renew Energy 141:613–631. https://doi.org/10.1016/J.RENENE.2019.04.020
Acknowledgements
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant no. 308515/2020-0, no. 306010/2021-6,) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) for the scholarships, and Universidade do Vale do Taquari – Univates, CNPq (grant no. 405688/2021-0) and FAPERGS (grant no. 19/2551-0001740-5, no. 20/2551-0000524-0, no. 22/2551-0000397-4 (Bioprocess and Biotechnology for Food Research Center—Biofood)) for the financial support granted for this research paper. We also thank Quatro G Pesquisa & Desenvolvimento Ltda for their support in the experiment.
Author information
Authors and Affiliations
Contributions
AG: Conceptualization, investigation, methodology, formal analysis, writing—original draft preparation, and writing—reviewing and editing. RS: Investigation, and methodology. GR: Conceptualization, methodology, formal analysis, writing—original draft preparation, and writing—reviewing and editing. JMC: Resources, and supervision. GV: Conceptualization, formal analysis, resources, supervision, writing—original draft preparation, and writing—reviewing and editing. CFVS: Conceptualization, investigation, methodology, formal analysis, resources, supervision, writing—original draft preparation, and writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they do not have any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gennari, A., Simon, R., Renard, G. et al. Lactose hydrolysis in packed-and fluidized-bed reactors using a recombinant β-galactosidase immobilized on magnetic core-shell capsules. Bioprocess Biosyst Eng 47, 263–273 (2024). https://doi.org/10.1007/s00449-023-02960-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02960-8