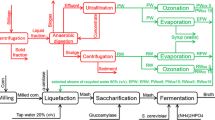
Abstract
Direct reutilization of condensate can inhibit ethanol fermentation, 2-phenylethyl alcohol and furfural existed in the condensate are considered to be inhibitors. To achieve the reutilization of the condensate, the ozonation combined with ion-exchange method was used. The results showed that the elimination rates of 2-phenylethyl alcohol and furfural reached 98.0% and 100.0%, respectively after ozonation, while the concentrations of acetic acid, propionic acid, butyric acid and valeric acid increased by 14.9%, 7.7%, 35.3% and 25.5%, respectively. The fermentation results showed that the inhibition of the condensate after ozonation was alleviated but was not completely eliminated. When the effluent volume treated by the ion-exchange method reached 80 BV, the concentrations of acetic acid, propionic acid, butyric acid and valeric acid decreased by 25.8%, 8.6%, 6.5% and 34.4%, respectively. The fermentation results showed that the inhibition of the condensate was completely eliminated after ozonation combined with ion-exchange treatment.



Similar content being viewed by others
References
Couallier EM, Payot T, Bertin AP et al (2006) Recycling of distillery effluents in alcoholic fermentation. Appl Biochem Biotechnol 133:217–237. https://doi.org/10.1385/ABAB:133:3:217
Maiorella B, Blanch HW, Wilke CR (1983) By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng 25:103–121. https://doi.org/10.1002/bit.260250109
Sagne C, Fargues C, Lewandowski R et al (2008) Screening of reverse osmosis membranes for the treatment and reuse of distillery condensates into alcoholic fermentation. Desalination 219:335–347. https://doi.org/10.1016/j.desal.2007.05.020
Fargues C, Lewandowski R, Lameloise M-L (2010) Evaluation of ion-exchange and adsorbent resins for the detoxification of beet distillery effluents. Ind Eng Chem Res 49:9248–9257. https://doi.org/10.1021/ie100330y
Lameloise M-L, Gavach M, Bouix M et al (2015) Combining reverse osmosis and ion-exchange allows beet distillery condensates to be recycled as fermentable dilution water. Desalination 363:75–81. https://doi.org/10.1016/j.desal.2014.09.036
Pant D, Adholeya A (2007) Biological approaches for treatment of distillery wastewater: a review. Bioresour Technol 98:2321–2334. https://doi.org/10.1016/j.biortech.2006.09.027
Zhang CM, Mao ZG, Wang X et al (2010) Effective ethanol production by reutilizing waste distillage anaerobic digestion effluent in an integrated fermentation process coupled with both ethanol and methane fermentations. Bioprocess Biosyst Eng 33:1067–1075. https://doi.org/10.1007/s00449-010-0432-8
Xu P, Janex M-L, Savoye P et al (2002) Wastewater disinfection by ozone: main parameters for process design. Water Res 36:1043–1055. https://doi.org/10.1016/S0043-1354(01)00298-6
Acero JL, Stemmler K, Gunten UV (2000) Degradation kinetics of atrazine and its degradation products with ozone and OH radicals: a predictive tool for drinking water treatment. Environ Sci Technol 34:591–597. https://doi.org/10.1021/es990724e
Hewes CG, Davison RR (1971) Kinetics of ozone decomposition and reaction with organics in water. AIChE J 17:141–147. https://doi.org/10.1002/aic.690170129
Gardoni D, Vailati A, Canziani R (2012) Decay of ozone in water: a review. Ozone Sci Eng 34:233–242. https://doi.org/10.1080/01919512.2012.686354
Rozas O, Baeza C, Núñez K et al (2017) Organic micropollutants (OMPs) oxidation by ozone: Effect of activated carbon on toxicity abatement. Sci Total Environ 590–591:430–439. https://doi.org/10.1016/j.scitotenv.2016.12.120
Wert EC, Rosario-Ortiz FL, Drury DD et al (2007) Formation of oxidation byproducts from ozonation of wastewater. Water Res 41:1481–1490. https://doi.org/10.1016/j.watres.2007.01.020
Narendranath NV, Thomas KC, Ingledew WM (2001) Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biotechnol 26:171–177. https://doi.org/10.1038/sj.jim.7000090
Beckner M, Ivey ML, Phister TG (2011) Microbial contamination of fuel ethanol fermentations. Lett Appl Microbiol 53:387–394. https://doi.org/10.1111/j.1472-765X.2011.03124.x
Yang XC, Wang K, Zhang JH et al (2016) Effect of acetic acid in recycling water on ethanol production for cassava in an integrated ethanol-methane fermentation process. Water Sci Technol 74:2392–2398. https://doi.org/10.2166/wst.2016.228
Zhang CM, Du FG, Wang X et al (2012) Effect of propanoic acid on ethanol fermentation by Saccharomyces cerevisiae in an ethanol-methane coupled fermentation process. Chin J Chem Eng 20:942–949. https://doi.org/10.1016/S1004-9541(12)60422-4
Li X, Wang YJ, Wang B et al (2019) Combination of ozonation and electrolysis process to enhance elimination of thirty structurally diverse pharmaceuticals in aqueous solution. J Hazard Mater 368:281–291. https://doi.org/10.1016/j.jhazmat.2019.01.062
Dubber D, Gray NF (2010) Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J Environ Sci Health A Tox Hazard Subst Environ Eng 45:1595–1600. https://doi.org/10.1080/10934529.2010.506116
Pampulha ME, Loureiro-Dias MC (2000) Energetics of the effect of acetic acid on growth of Saccharomyces cerevisiae. FEMS Microbiol Lett 184:69–72. https://doi.org/10.1111/j.1574-6968.2000.tb08992.x
Acknowledgements
The research leading to these results received funding from [National Natural Science Foundation of China] under Grant Agreement No [21506075].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sheng, X., Li, Z. & Zhang, J. Exploring the resourcing technology of condensate using ozonation combined with ion-exchange in ethanol fermentation. Bioprocess Biosyst Eng 45, 1919–1926 (2022). https://doi.org/10.1007/s00449-022-02782-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02782-0