Abstract
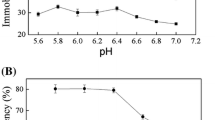
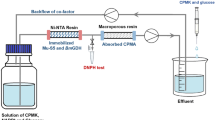
N-Acetylglucosamine-2-epimerase (AGE) and N-acetylneuraminic acid lyase (NAL) were immobilized for synthesis of N-acetylneuraminic acid (Neu5Ac) on three resins: Amberzyme oxirane resin (AOR), poly (styrene-co-DVB)-Br resin (PBR) and amino resin (AR). The loading capacity and immobilized enzyme activity showed that AR was the best carrier. Three methods of glutaraldehyde cross-linking were tested and simultaneous cross-linking and immobilization was demonstrated to be the best method. The functional properties of immobilized AGE and NAL were studied and compared to those of the free enzyme. The highest enzyme activities of free and immobilized AGE were obtained in 0.1 M potassium phosphate buffer at pH 7.5 and a temperature of 37 °C. Comparatively, the highest NAL activities were at pH 8.5. Meanwhile, an increase in K m (from 1.14 to 1.31 mg·mL−1 for AGE and from 1.05 to 1.25 mg·mL−1 for NAL) and a decrease in V max (from 177.53 to 106.37 µg·min−1 mL−1 for AGE and from 126.41 to 95.96 µg·min−1 mL−1 for NAL) were recorded after immobilization. The AR–glutaraldehyde–enzyme system exhibited better thermal stability than the free enzyme, and retained 72% of its initial activity even after eight repeated runs. The apparent activation energy (E a) of the free and immobilized AGE (NAL) was 117.14 kJ·mol−1 (124.21 kJ·mol−1) and 78.45 kJ·mol−1 (66.64 kJ·mol−1), respectively, implying that the catalytic efficiency of the immobilized enzyme was restricted by mass-transfer rather than kinetic limit. Subsequently, Neu5Ac production from GlcNAc using immobilized enzymes in one reactor was carried out resulting 101.45 g·L−1 of Neu5Ac and the highest conversion ratio of 82%. This method of enzyme immobilization may have a promising future for Neu5Ac production in industry.








Similar content being viewed by others
References
Schauer R (2000) Achievements and challenges of sialic acid research. Glycoconj J 17:485–499
Vonitzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, Phan TV, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron JM, Penn CR (1993) Rational design of potent sialidase-based inhibitors of influenza-virus replication. Nature 363:418–423
Maru I, Ohnishi J, Ohta Y, Tsukada Y (2002) Why is sialic acid attracting interest now? Complete enzymatic synthesis of sialic acid with N-acylglucosamine 2-epimerase. J Biosci Bioeng 93:258–265
Shamberger RJ (1984) Serum sialic acid in normals and in cancer patients. J Clin Chem Clin Biochem 22:647–651
Saez JJ, Senravarela A (1995) Evaluation of lipid-bound sialic acid (Lsa) as a tumor-marker. Int J Biol Marker 10:174–179
Nilsson KGI (1989) Enzymic-synthesis of di-saccharide and trisaccharide glycosides, using glycosidases and beta-d-galactoside 3-alpha-sialyl-transferase. Carbohydr Res 188:9–17
Maru I, Ohta Y, Murata K, Tsukada Y (1996) Molecular cloning and identification of N-acyl-d-glucosamine 2-epimerase from porcine kidney as a renin-binding protein. J Biol Chem 271:16294–16299
Tabata K, Koizumi S, Endo T, Ozaki A (2002) Production of N-acetyl-d-neuraminic acid by coupling bacteria expressing N-acetyl-d-glucosamine 2-epimerase and N-acetyl-d-neuraminic acid synthetase. Enzyme Microb Technol 30:327–333
Lee YC, Chien HC, Hsu WH (2007) Production of N-acetyl-d-neuraminic acid by recombinant whole cells expressing Anabaena sp. CH1 N-acetyl-d-glucosamine 2-epimerase and Escherichia coli N-acetyl-d-neuraminic acid lyase. J Biotechnol 129:453–460
Kragle U, Gygax D, Ghisalba O, Wandrey C (1991) Enzymatic two-step synthesis of N-acetyl-neuraminic acid in the enzyme membrane reactor. Angew Chem Int Ed Engl 30:827–828
Maru I, Ohnishi J, Ohta Y, Tsukada Y (1998) Simple and large-scale production of N-acetylneuraminic acid from N-acetyl-d-glucosamine and pyruvate using N-acyl-d-glucosamine 2-epimerase and N-acetylneuraminate lyase. Carbohydr Res 306:575–578
Lin BX, Zhang ZJ, Liu WF, Dong ZY, Tao Y (2013) Enhanced production of N-acetyl-d-neuraminic acid by multi-approach whole-cell biocatalyst. Appl Microbilo Biotechnol 97:4775–4784
Yang JB, Ni KF, Wei DZ, Ren YH (2015) One-step purification and immobilization of his-tagged protein via Ni2+-functionalized Fe3O4 @ polydopamine magnetic nanoparticles. Biotechnol Bioprocess Eng 20:901–907
Pal A, Khanum F (2011) Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: characterization of immobilized enzyme. Process Biochem 46:1315–1322
White CA, Kennedy JF (1980) Popular matrices for enzyme and other immobilizations. Enzyme Microb Technol 2:82–90
Ghiaci M, Aghaei H, Soleimanian S, Sedaghat ME (2009) Enzyme immobilization. Part 2. Immobilization of alkaline phosphatase on Na-bentonite and modified bentonite. Appl Clay Sci 43:308–316
Lai JK, Chuang TH, Jan JS, Wang SSS (2010) Efficient and stable enzyme immobilization in a block copolypeptide vesicle-templated biomimetic silica support. Colloids Surf B 80:51–58
Panzavolta F, Soro S, Amato RD, Palocci C, Cernia E, Russo MV (2005) Acetylenic polymers as new immobilization matrices for lipolytic enzymes. J Mol Catal B Enzym 32:67–76
Kharrat N, Ali YB, Marzouk S, Gargouri YT, Karra-Chaabouni M (2011) Immobilization of Rhizopus oryzae lipase on silica aerogels by absorption: comparison with the free enzyme. Process Biochem 46:1083–1089
Olson AC, Stanley WL (1973) Lactase and other enzymes bound to a phenolformaldehyde resin with glutaraldehyde. J Agric Food Chem 21:440–445
Zhang Y, Zhang Y, Jiang J, Li L, Yu C, Hei T (2011) Surface derivatization with spacer molecules on glutaraldehyde-activated amino-microplates for covalent immobilization of β-glucosidase. Appl Surf Sci 257:2712–2716
Sun WJ, Ji WY, Li N, Tong P, Cheng J (2013) Construction and expression of a polycistronic plasmid encoding N-acetylglucosamine 2-epimerase and N-acetylneuraminic acid lyase simultaneously for production of N-acetylneuraminic acid. Bioresour Technol 130:23–29
Hu SY, Chen J, Yang ZY, Shao LJ, Bai H, Luo JL, Jiang WH, Yang YL (2010) Coupled bioconversion for preparation of N-acetyl-d-neuraminic acid using immobilized N-acetyl-d-glucosamine-2-epimerase and N-acetyl-d-neuraminic acid lyase. Appl Microbiol Biotechnol 85:1383–1391
Bradford A (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Ortega N, Perez-Mateos M, Pilar MC, Busto MD (2009) Neutrase immobilization on alginate-glutaraldehyde beads by covalent attachment. J Agric Food Chem 57:109–115
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–802
Wei L, Zhang W, Lu H, Yang P (2010) Immobilization of enzyme on detonation nanodiamond for highly efficient proteolysis. Talanta 80:1298–1304
Heitmann T, Wenzig E, Mersmann A (1997) Characterization of three different potato starches and kinetics of their enzymatic hydrolysis by an alpha-amylase. Enzyme Microb Technol 20:259–267
Shi LE, Tang ZX, Yi Y, Chen JS, Wang H, Xiong WY (2010) Study of immobilization of nuclease P1 on paper cellulose. Biotechnol Biotechnol Equip 24:1997–2003
Pundir CS, Bhambi M, Chauhan NS (2009) Chemical activation of egg shell membrane for covalent immobilization of enzymes and its evaluation as inert support in urinary oxalate determination. Talanta 77:1688–1693
Karout A, Chopard C, Pierre AC (2007) Immobilization of a lipoxygenase in silica gels for application in aqueous media. J Mol Catal B Enzym 44:117–127
Ye P, Wang RB, Wang XP (2009) Quantitative enzyme immobilization: control of the carboxyl group density on support surface. J Mol Catal B Enzym 61:296–302
Karra-Chaabouni M, Bouaziz I, Boufi S, Rego AMB, Gargouri Y (2008) Physical immobilization of Rhizopus oryzae lipase onto cellulose substrate: activity and stability studies. Colloids Surf B 66:168–177
Mozhaev VV, Martinek K (1990) Structure–stability relationships in proteins: a guide to approaches to stabilizing enzymes. Adv Drug Delivery Rev 4:387–419
Mozhaev VV (1993) Mechanism-based strategies for protein thermostabilization. Trends Biotechnol 11:88–95
Mateo C, Grazu V, Palomo JM, Lopez-Gallego F, Fernandez-Lafuente R, Guisan JM (2007) Immobilization of enzymes on heterofunctional epoxy supports. Nat Protoc 2:1022–1033
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, 2013CB733602), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Major Research Plan of the National Natural Science Foundation of China (21390204), Program for Changjiang Scholars and Innovative Research Team in University (Grant No.: IRT_14R28), and Jiangsu National Synergistic Innovation Center for Advanced Materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cheng, J., Zhuang, W., Tang, C. et al. Efficient immobilization of AGE and NAL enzymes onto functional amino resin as recyclable and high-performance biocatalyst. Bioprocess Biosyst Eng 40, 331–340 (2017). https://doi.org/10.1007/s00449-016-1700-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1700-z