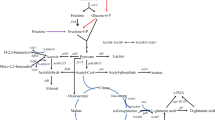
Abstract
The aims of this research were to screen and characterize a new microbial source of γ-PGA, to optimize aspects of culture conditions and medium composition using central composite design and response surface methodologies. The influence of bioreactor stirring rates on the production of γ-PGA was also investigated and the oxygen volumetric mass transfer coefficients (k La) were established. The most productive strain was identified by 16S rDNA analysis as Bacillus subtilis, and its γ-PGA production in rotatory shaker was threefold increased under optimized conditions (37 °C, pH 6.9, and 1.22 mM Zn2+), compared to conventional medium. In bioreactor, the γ-PGA production was further increased, reaching 17 g l−1, 70 % higher than shaker cultures. γ-PGA production showed high dependency on oxygen transfer. At k La of 210 h−1, the cultivation time could be reduced to 48 h, about 50 % of the time required for operations at k La 55 h−1.






Similar content being viewed by others
References
Shih IL, Van YT (2001) The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour Technol 79:207–225
Candela T, Fouet A (2006) Poly-gamma-glutamate in bacteria. Mol Microbiol 60:1091–1098
Buescher JM, Margaritis A (2007) Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit Rev Biotechnol 27:1–19
Manocha B, Margaritis A (2008) Production and characterization of gamma-polyglutamic acid nanoparticles for controlled anticancer drug release. Crit Rev Biotechnol 28:83–99
Sung MH, Park C, Kim CJ, Poo H, Soda K, Ashiuchi M (2005) Natural and edible biopolymer poly-gamma-glutamic acid: synthesis, production, and applications. Chem Rec 5:352–366
Taniguchi M, Kato K, Shimauchi A, Xu P, Nakayama H, Fujita KI, Tanaka T, Tarui Y, Hirasawa E (2005) Proposals for wastewater treatment by applying flocculating activity of cross-linked poly-gamma-glutamic acid. J Biosci Bioeng 99:245–251
Carvajal-Zarrabal O, Nolasco-Hipólito C, Barradas-Dermitz DM, Hayward-Jones PM, Aguilar-Uscanga MG, Bujang K (2012) Treatment of vinasse from tequila production using polyglutamic acid. J Environ Manag 95:S66–S70
Bajaj I, Singhal R (2011) Poly (glutamic acid)–an emerging biopolymer of commercial interest. Bioresour Technol 102:5551–5561
Schallmey M, Singh A, Ward OP (2004) Developments in the use of Bacillus species for industrial production. Can J Microbiol 50:1–17
Oppermann-Sanio FB, Steinbuchel A (2002) Occurrence, functions and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften 89:11–22
Ashiuchi M, Misono H (2002) Biochemistry and molecular genetics of poly-gamma-glutamate synthesis. Appl Microbiol Biotechnol 59:9–14
Ashiuchi M, Kamei T, Baek DH, Shin SY, Sung MH, Soda K, Yagi T, Misono H (2001) Isolation of Bacillus subtilis (chungkookjang), a poly-gamma-glutamate producer with high genetic competence. Appl Microbiol Biotechnol 57:764–769
Ashiuchi M, Shimanouchi K, Nakamura H, Kamei T, Soda K, Park C, Sung M-H, Misono H (2004) Enzymatic synthesis of high-molecular-mass poly-{gamma}-glutamate and regulation of its stereochemistry. Appl Environ Microbiol 70:4249–4255
Kunioka M (2004) Biodegradable Water Absorbent Synthesized from Bacterial Poly(amino acid)s. Macromol Biosci 4:324–329
Hoppensack A, Oppermann-Sanio FB, Steinbüchel A (2003) Conversion of the nitrogen content in liquid manure into biomass and polyglutamic acid by a newly isolated strain of Bacillus licheniformis. FEMS Microbiol Lett 218:39–45
Mahmoud DAR (2006) Isolation of polyglutamic acid flocculant producing bacteria from extreme Egyptian environments. J Appl Sci Res 2:608–612
Soliman N, Berekaa M, Abdel-Fattah Y (2005) Polyglutamic acid (PGA) production by Bacillus sp. SAB-26: application of Plackett–Burman experimental design to evaluate culture requirements. Appl Microbiol Biotechnol 69:259–267
Xu H, Jiang M, Li H, Lu D, Ouyang P (2005) Efficient production of poly([gamma]-glutamic acid) by newly isolated Bacillus subtilis NX-2. Process Biochem 40:519–523
Cao M, Geng W, Liu L, Song C, Xie H, Guo W, Jin Y, Wang S (2011) Glutamic acid independent production of poly-γ-glutamic acid by Bacillus amyloliquefaciens LL3 and cloning of pgsBCA genes. Bioresour Technol 102:4251–4257
Zhang H, Zhu J, Zhu X, Cai J, Zhang A, Hong Y, Huang J, Huang L, Xu Z (2012) High-level exogenous glutamic acid-independent production of poly-(γ-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresour Technol
Leonard CG, Housewright RD, Thorne CB (1958) Effects of some metallic ions on glutamyl polypeptide synthesis by Bacillus subtilis. J Bacteriol 76:499–503
Do JH, Chang HN, Lee SY (2001) Efficient recovery of gamma-poly (glutamic acid) from highly viscous culture broth. Biotechnol Bioeng 76:219–223
Shih IL, Van YT, Yeh LC, Lin HG, Chang YN (2001) Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour Technol 78:267–272
Shih IL, Van YT, Chang YN (2002) Application of statistical experimental methods to optimize production of poly(gamma-glutamic acid) by Bacillus licheniformis CCRC 12826. Enzyme Microb Technol 31:213–220
Shih I-L, Yu Y-T (2005) Simultaneous and selective production of levan and poly(γ-glutamic acid) by Bacillus subtilis. Biotechnol Lett 27:103–106
Cromwick AM, Birrer GA, Gross RA (1996) Effects of pH and aeration on gamma-poly(glutamic acid) formation by Bacillus licheniformis in controlled batch fermenter cultures. Biotechnol Bioeng 50:222–227
Du G, Yang G, Qu Y, Chen J, Lun S (2005) Effects of glycerol on the production of poly([gamma]-glutamic acid) by Bacillus licheniformis. Process Biochem 40:2143–2147
Yoon SH, Hwan Do J, Yup Lee S, Nam Chang H (2000) Production of poly-γ-glutamic acid by fed-batch culture of Bacillus licheniformis. Biotechnol Lett 22:585–588
Richard A, Margaritis A (2003) Rheology, oxygen transfer, and molecular weight characteristics of poly(glutamic acid) fermentation by Bacillus subtilis. Biotechnol Bioeng 82:299–305
Bajaj IB, Singhal RS (2009) Enhanced production of poly (gamma-glutamic acid) from Bacillus licheniformis NCIM 2324 by using metabolic precursors. Appl Biochem Biotechnol 159:133–141
Kirschner P, Meier A, Böttger EC (1993) Genotypic identification and detection of mycobacteria: facing novel and uncultured pathogens. In: Persing DH, Smith TF, Tenover FC, White TJ (eds) Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington (DC)
Sneath PHA (1986) Endospore-forming gram-positive rods and cocci. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s Manual of Systematic Bacteriology. Williams & Wilkins, Baltimore
MacFadin JF (ed) (1980) Biochemical test for identification of medical bacteria, 2nd edn. Williams & Wilkins, Baltimore
Zwietering MH, Jongenburger I, Rombouts FM, Vantriet K (1990) Modeling of the Bacterial-Growth Curve. Appl Environ Microbiol 56:1875–1881
Doran PM (1995) Bioprocess engineering principles. Academic Press, London
Kanno A, Takamatsu H (1995) Determination of polyglutamic acid in “Natto” using cetyltrimethylammonium bromide (Studies on “Natto” part V). Nippon Shokuhin Kagaku Kogaku Kaishi 42:878–886
Ashiuchi M (2011) Analytical approaches to poly-γ-glutamate: quantification, molecular size determination, and stereochemistry investigation. J Chromatogr B 879:3096–3101
Porwal S, Lal S, Cheema S, Kalia VC (2009) Phylogeny in aid of the present and novel microbial lineages: diversity in Bacillus. PLoS One 4:e4438
Xu D, Cote JC (2003) Phylogenetic relationships between Bacillus species and related genera inferred from comparison of 3′ end 16S rDNA and 5′ end 16S-23S ITS nucleotide sequences. Int J Syst Evol Microbiol 53:695–704
Sumpavapol P, Tongyonk L, Tanasupawat S, Chokesajjawatee N, Luxanani P, Visessanguan W (2010) Bacillus siamensis sp. nov., isolated from salted crab (poo-khem) in Thailand. Int J Syst Evol Microbiol 60:2364–2370
Priest FG, Goodfellow M, Todd C (1988) A Numerical Classification of the Genus Bacillus. J Gen Microbiol 134:1847–1882
Reva ON, Sorokulova IB, Smirnov VV (2001) Simplified technique for identification of the aerobic spore-forming bacteria by phenotype. Int J Syst Evol Microbiol 51:1361–1371
Welker NE, Campbell LL (1967) Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol 94:1124–1130
Kim KM, Kim MJ, Kim DH, Park YS, Kang JS (2009) Characterization of Bacillus polyfermenticus KJS-2 as a Probiotic. J Microbiol Biotechnol 19:1013–1018
Richard A, Margaritis A (2003) Optimization of cell growth and poly(glutamic acid) production in batch fermentation by Bacillus subtilis. Biotechnol Lett 25:465–468
Wu Q, Xu H, Ying HJ, Ouyang PK (2010) Kinetic analysis and pH-shift control strategy for poly(gamma-glutamic acid) production with Bacillus subtilis CGMCC 0833. Biochem Eng J 50:24–28
Chen X, Chen S, Sun M, Yu Z (2005) High yield of poly-[gamma]-glutamic acid from Bacillus subtilis by solid-state fermentation using swine manure as the basis of a solid substrate. Bioresour Technol 96:1872–1879
Chen X, Chen S, Sun M, Yu Z (2005) Medium optimization by response surface methodology for poly-γ-glutamic acid production using dairy manure as the basis of a solid substrate. Appl Microbiol Biotechnol 69:390–396
Bajaj IB, Singhal RS (2010) Effect of aeration and agitation on synthesis of poly (gamma-glutamic acid) in batch cultures of Bacillus licheniformis NCIM 2324. Biotechnol Bioprocess Eng 15:635–640
García-Ochoa F, Gomez E, Santos VE, Merchuk JC (2010) Oxygen uptake rate in microbial processes: an overview. Biochem Eng J 49:289–307
García-Ochoa F, Castro EG, Santos VE (2000) Oxygen transfer and uptake rates during xanthan gum production. Enzyme Microb Technol 27:680–690
Bandaiphet C, Prasertsan P (2006) Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr Polym 66:216–228
Chun BH, Lee YK, Chung N (2012) Poly-γ-glutamic acid enhances the growth and viability of Chinese hamster ovary cells in serum-free medium. Biotechnol Lett pp 1–4
García-Ochoa F, Santos VE, Casas JA, Gomez E (2000) Xanthan gum: production, recovery, and properties. Biotechnol Adv 18:549–579
Casas JA, Santos VE, Garcia-Ochoa F (2000) Xanthan gum production under several operational conditions: molecular structure and rheological properties. Enzyme Microb Technol 26:282–291
Giavasis I, Harvey LM, McNeil B (2006) The effect of agitation and aeration on the synthesis and molecular weight of gellan in batch cultures of Sphingomonas paucimobilis. Enzyme Microb Technol 38:101–108
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, S.B., Cantarelli, V.V. & Ayub, M.A.Z. Production and optimization of poly-γ-glutamic acid by Bacillus subtilis BL53 isolated from the Amazonian environment. Bioprocess Biosyst Eng 37, 469–479 (2014). https://doi.org/10.1007/s00449-013-1016-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1016-1