Abstract
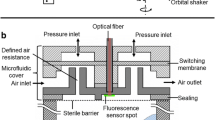
A new online monitoring technique to measure the physiological parameters, dissolved oxygen (DO) and pH of microbial cultures in continuously shaken 24-well microtiter plates (MTP) is introduced. The new technology is based on immobilised fluorophores at the bottom of standard 24-well MTPs. The sensor MTP is installed in a sensor dish reader, which can be fixed on an orbital shaker. This approach allows real online measurements of physiological parameters during continuous shaking of cultures without interrupting mixing and mass transfer like currently available technologies do. The oxygen transfer conditions at one constant shaking frequency (250 1/min) and diameter (25 mm) was examined with the chemical sulphite oxidation method. Varied filling volumes (600–1,200 μL) of Escherichia coli cultures demonstrated the importance of sufficient oxygen transfer to the culture. Cultures with higher filling volumes were subjected to an oxygen limitation, which influenced the cell metabolism and prolongated the cultivation time. The effects could be clearly monitored by online DO and pH measurements. A further study of different media in an E. coli fermentation elucidated the different growth behaviour in response to the medium composition. The MTP fermentations correlated very well with parallel fermentations in shake flasks. The new technique gives valuable new insights into biological processes at a very small scale, thus enabling parallel experimentation and shorter development times in bioprocessing.




Similar content being viewed by others
References
Lye GJ, Dalby PA, Woodley JM (2002) Better biocatalytic processes faster: New tools for the implementation of biocatalysis in organic synthesis. Org Process Res Dev 6: 434–440
Boettner M, Prinz B, Holz C, Stahl U, Lang C (2002) High-throughput screening for expression of heterologous proteins in the yeast Pichia pastoris. J Biotechnol 99: 51–62
Kumar S, Wittmann C, Heinzle E (2004) Minibioreactors. Biotechnol Lett 26: 1–10
Hermann R, Walther N, Maier U, Büchs J (2001) Optical method for the determination of the oxygen-transfer capacity of small bioreactors based on sulfite oxidation. Biotechnol Bioeng 74: 355–363
Hermann R, Lehmann M, Büchs J (2003) Characterization of gas-liquid mass transfer phenomena in microtiter plates. Biotechnol Bioeng 81: 178–186
Weiss S, John GT, Klimant I, Heinzle E (2002) Modeling of mixing in 96-well microplates observed with fluorescence indicators. Biotechnol Progr 18: 821–830
John GT, Klimant I, Wittmann C, Heinzle E (2003) Integrated optical sensing of dissolved oxygen in microtiter plates: A novel tool for microbial cultivation. Biotechnol Bioeng 81: 829–836
Bambot SB, Holavanahali R, Lakowicz JR, Carter GM, Rao G (1994) Phase fluorometric sterilizable optical oxygen sensor. Biotechnol Bioeng 43: 1139–1145
Huber C, Klimant I, Krause C, Werner T, Mayr T, Wolfbeis OS (2000) Optical sensor for seawater salinity. Fresen J Anal Chem 368: 196–202
Holst G, Kohls O, Klimant I, Konig B, Kuhl M, Richter T (1998) A modular luminescence lifetime imaging system for mapping oxygen distribution in biological samples. Sensor Actuat B-Chem 51: 163–170
Hartmann P, Ziegler W, Holst G, Lubbers DW (1997) Oxygen flux fluorescence lifetime imaging. Sensor Actuat B-Chem 38: 110–115
Kosch U, Klimant I, Werner T, Wolfbeis OS (1998) Strategies to design pH optodes with luminescence decay times in the microsecond time regime. Anal Chem 70: 3892–3897
John GT, Goelling D, Klimant I, Schneider H, Heinzle E (2003) PH-sensing 96-well microtitre plates for the characterization of acid production by dairy starter cultures. J Dairy Res 70: 327–333
Linek V, Benes P, Vacek V (1989) Dynamic pressure method for kLa measurement in large-scale bioreactors. Biotechnol Bioeng 33: 1406–1412
Weisenberger S, Schumpe A (1996) Estimation of gas solubilities in salt solutions at temperatures from 273 K to 363 K. Aiche J 42: 298–300
Akita K (1981) Diffusivities of gases in aqueous-electrolyte solutions. Ind Eng Chem Fund 20: 89–94
Kensy F, Zimmermann HF, Knabben I, Anderlei T, Trauthwein H, Dingerdissen U, Büchs J (2005) Oxygen transfer phenomena in 48-well microtiter plates: determination by optical monitoring of sulfite oxidation and verification by real-time measurement during microbial growth. Biotechnol Bioeng 89: 698–708
Duetz WA, Witholt B (2001) Effectiveness of orbital shaking for the aeration of suspended bacterial cultures in square-deepwell microtiter plates. Biochem Eng J 7: 113–115
Maier U, Losen M, Büchs J (2004) Advances in understanding and modeling the gas-liquid mass transfer in shaking flasks. Biochem Eng J 17: 155–168
Wilms B, Hauck A, Reuss M, Syldatk C, Mattes R, Siemann M, Altenbuchner J (2001) High-cell-density fermentation for production of L-N-carbamoylase using an expression system based on the Escherichia coli rhaBAD promoter. Biotechnol Bioeng 73: 95–103
Anderlei T, Büchs J (2001) Device for sterile online measurement of the oxygen transfer rate in shaking flasks. Biochem Eng J 7: 157–162
Anderlei T, Zang W, Büchs J (2004) Online respiration activity measurement (OTR, CTR, RQ) in shake flasks. Biochem Eng J 17: 187–194
Losen M, Fröhlich B, Pohl M, Büchs J (2004) Effect of oxygen limitation and medium composition on Escherichia coli fermentation in shake-flask cultures. Biotechnol Progr 20: 1062–1068
Alfoldi L, Rasko II (1970) L-serine deaminating enzymes in Escherichia coli crude extracts. FEBS Lett 6: 73–76
Robbins JW, Taylor KB (1989) Optimization of Escherichia coli growth by controlled addition of glucose. Biotechnol Bioeng 34: 1289–1294
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kensy, F., John, G.T., Hofmann, B. et al. Characterisation of operation conditions and online monitoring of physiological culture parameters in shaken 24-well microtiter plates. Bioprocess Biosyst Eng 28, 75–81 (2005). https://doi.org/10.1007/s00449-005-0010-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-005-0010-7