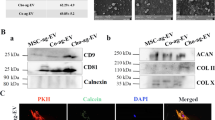
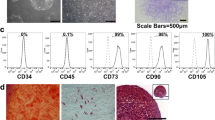
Abstract
Extracellular vesicles (EVs) may have a key therapeutic role and offer an innovative treatment for osteoarthritis (OA). Studies have shown that ratio of MSC/chondrocyte could affect their therapeutic outcomes. Here, we investigate the chondrogenic potential and therapeutic effect of EVs derived from MSCs and chondrocytes in the naïve, chondrogenically primed, and co-culture states to treat OA. EVs are isolated from naïve MSCs (M-EV), chondrogenically primed MSCs (cpM-EV), chondrocytes (C-EV), and co-cultures of chondrocytes plus MSCs at ratios of 1:1 (C/M-EV), 2:1 (2C/M-EV), and 4:1 (4C/M-EV). We characterized the isolated EVs in terms of surface markers, morphology, size, and zeta potential, and evaluated their chondrogenic potential in vitro by qRT-PCR and histological analyses. Next, these EVs were intra-articularly injected into osteoarthritic cartilage of a rat model and assessed by radiography, gait parameters, and histological and immunohistochemical analyses. EVs obtained from chondrocytes co-cultured with MSCs resulted in improved matrix production and functional differentiation. Our research showed that close proximity between the two cell types was essential for this response, and improved chondrogenesis and matrix formation were the outcomes of this interaction in vitro. Furthermore, in the in vivo rat OA model induced by a monoiodoacetate (MIA), we observed recovery from OA by increasing ratio of the C/M-derived EV group compared to the other groups. Our findings show that the increasing chondrocyte ratio to MSC leads to high chondrogenic induction and the therapeutic effect of harvested EVs for cartilage repair.






Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding authors.
Abbreviations
- AC:
-
Articular cartilage
- ACAN:
-
Aggrecan
- Cho:
-
Chondrocyte
- Col II:
-
Collagen type II
- Col X:
-
Collagen type X
- cpM:
-
Chondrogenically primed MSCs
- DLS:
-
Dynamic light scattering
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ECM:
-
The extracellular matrix
- EVs:
-
Extracellular vesicles
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase (endogenous control gene)
- H&E:
-
Hematoxylin and eosin
- HRP:
-
Horseradish peroxidase
- IHC:
-
Immunohistochemistry
- IP:
-
Intraperitoneal
- IOD:
-
Integrated optical density
- MIA:
-
Monoiodoacetate
- MISEV:
-
The first Minimal Information for Studies of Extracellular Vesicles
- MSCs:
-
Mesenchymal stem cells
- NBF:
-
Neutral buffered formalin
- OA:
-
Osteoarthritis
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- SEM:
-
Scanning electron microscopy
- TGF-β3:
-
Transforming growth factor-β3
- UC:
-
Ultracentrifugation
References
Ahmed N, Dreier R, Gopferich A, Grifka J, Grassel S (2007) Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem 20:665–678
Akers JC, Gonda D, Kim R, Carter BS, Chen CC (2013) Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 113:1–11
Angeby Moller K, Kinert S, Storkson R, Berge OG (2012) Gait analysis in rats with single joint inflammation: influence of experimental factors. PLoS One 7:e46129
Ayub H, Clare M, Milic I, Chmel NP, Boning H, Devitt A, Krey T, Bill RM, Rothnie AJ (2020) CD81 extracted in SMALP nanodiscs comprises two distinct protein populations within a lipid environment enriched with negatively charged headgroups. Biochim Biophys Acta Biomembr 1862:183419
Beckett J, Jin W, Schultz M, Chen A, Tolbert D, Moed BR, Zhang Z (2012) Excessive running induces cartilage degeneration in knee joints and alters gait of rats. J Orthop Res 30:1604–1610
Beit-Yannai E, Tabak S, Stamer WD (2018) Physical exosome:exosome interactions. J Cell Mol Med 22:2001–2006
Bian L, Zhai DY, Mauck RL, Burdick JA (2011) Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 17:1137–1145
Boettger MK, Weber K, Schmidt M, Gajda M, Brauer R, Schaible HG (2009) Gait abnormalities differentially indicate pain or structural joint damage in monoarticular antigen-induced arthritis. Pain 145:142–150
Boker KO, Lemus-Diaz N, Rinaldi Ferreira R, Schiller L, Schneider S, Gruber J (2018) The impact of the CD9 tetraspanin on lentivirus infectivity and exosome secretion. Mol Ther 26:634–647
Chen S, Fu P, Cong R, Wu H, Pei M (2015) Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis 2:76–95
Chen Y, Xue K, Zhang X, Zheng Z, Liu K (2018) Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res Ther 9:318
Cho H, Kim D, Kim K (2018) Engineered co-culture strategies using stem cells for facilitated chondrogenic differentiation and cartilage repair. Biotechnol Bioprocess Eng 23:261–270
Cosenza S, Ruiz M, Maumus M, Jorgensen C, Noel D (2017) Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem cell-derived vesicles. Int J Mol Sci 18
Edd SN, Bennour S, Ulrich B, Jolles BM, Favre J (2020) Modifying stride length in isolation and in combination with foot progression angle and step width can improve knee kinetics related to osteoarthritis: a preliminary study in healthy subjects. J Biomech Eng 142
Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ (1998) Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 273:20121–20127
Feng C, Luo X, He N, Xia H, Lv X, Zhang X, Li D, Wang F, He J, Zhang L, Lin X, Lin L, Yin H, He J, Wang J, Cao W, Wang R, Zhou G, Wang W (2018) Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model. Tissue Eng Part A 24:219–233
Fischer J, Dickhut A, Rickert M, Richter W (2010) Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum 62:2696–2706
Gao G, Fan C, Li W, Liang R, Wei C, Chen X, Yang Y, Zhong Y, Shao Y, Kong Y, Li Z, Zhu X (2021) Mesenchymal stem cells: ideal seeds for treating diseases. Hum Cell 34:1585–1600
He L, He T, Xing J, Zhou Q, Fan L, Liu C, Chen Y, Wu D, Tian Z, Liu B, Rong L (2020) Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther 11:276
Hosseini S, Taghiyar L, Safari F, Baghaban Eslaminejad M (2018) Regenerative medicine applications of mesenchymal stem cells. In: Turksen K (ed) Cell biology and translational medicine, vol 2. approaches for diverse diseases and conditions. Springer International Publishing, Cham, pp 115–141
Hosseinzadeh M, Kamali A, Hosseini S, Baghaban Eslaminejad M (2021) Higher chondrogenic potential of extracellular vesicles derived from mesenchymal stem cells compared to chondrocytes-EVs in vitro. Biomed Res Int 2021:9011548
Huang CL, Liu D, Masuya D, Kameyama K, Nakashima T, Yokomise H, Ueno M, Miyake M (2004) MRP-1/CD9 gene transduction downregulates Wnt signal pathways. Oncogene 23:7475–7483
Katsuda T, Kosaka N, Takeshita F, Ochiya T (2013) The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 13:1637–1653
Kim JE, Song DH, Kim SH, Jung Y, Kim SJ (2018) Development and characterization of various osteoarthritis models for tissue engineering. PLoS One 13:e0194288
Kim M, Steinberg DR, Burdick JA, Mauck RL (2019) Extracellular vesicles mediate improved functional outcomes in engineered cartilage produced from MSC/chondrocyte cocultures. Proc Natl Acad Sci U S A 116:1569–1578
Knuth CA, Andres Sastre E, Fahy NB, Witte-Bouma J, Ridwan Y, Strabbing EM, Koudstaal MJ, van de Peppel J, Wolvius EB, Narcisi R, Farrell E (2019) Collagen type X is essential for successful mesenchymal stem cell-mediated cartilage formation and subsequent endochondral ossification. Eur Cell Mater 38:106–122
Krampera M, Pizzolo G, Aprili G, Franchini M (2006) Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone 39:678–683
Lettry V, Hosoya K, Takagi S, Okumura M (2010) Coculture of equine mesenchymal stem cells and mature equine articular chondrocytes results in improved chondrogenic differentiation of the stem cells. Jpn J Vet Res 58:5–15
Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3:26913
Mankin HJ, Dorfman H, Lippiello L, Zarins A (1971) Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 53:523–537
Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y (2018) Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther 9:247
Marolt Presen D, Traweger A, Gimona M, Redl H (2019) Mesenchymal stromal cell-based bone regeneration therapies: from cell transplantation and tissue engineering to therapeutic secretomes and extracellular vesicles. Front Bioeng Biotechnol 7:352
Mazzocca A, Liotta F, Carloni V (2008) Tetraspanin CD81-regulated cell motility plays a critical role in intrahepatic metastasis of hepatocellular carcinoma. Gastroenterology 135(244–256):e241
Meretoja VV, Dahlin RL, Kasper FK, Mikos AG (2012) Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials 33:6362–6369
Morais SV, Czeczko NG, Malafaia O, Ribas JMF, Garcia JB, Miguel MT, Zini C, Massignan AG (2016) Osteoarthritis model induced by intra-articular monosodium iodoacetate in rats knee. Acta Cir Bras 31:765–773
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD Jr, Thomson AW (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104:3257–3266
Mueller MB, Tuan RS (2008) Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58:1377–1388
Najar M, Krayem M, Merimi M, Burny A, Meuleman N, Bron D, Raicevic G, Lagneaux L (2018) Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm Res 67:467–477
Nasiri N, Hosseini S, Reihani-Sabet F, Baghaban Eslaminejad M (2022) Targeted mesenchymal stem cell therapy equipped with a cell-tissue nanomatchmaker attenuates osteoarthritis progression. Sci Rep 12:4015
Noronha NC, Mizukami A, Caliari-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR (2019) Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther 10:131
Park YB, Ha CW, Kim JA, Han WJ, Rhim JH, Lee HJ, Kim KJ, Park YG, Chung JY (2017) Single-stage cell-based cartilage repair in a rabbit model: cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthr Cartil 25:570–580
Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X (2016) Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci 12:836–849
Shi J, Zhao YC, Niu ZF, Fan HJ, Hou SK, Guo XQ, Sang L, Lv Q (2021) Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases: progress and prospect. World J Stem Cells 13:49–63
Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B (2011) Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B 87:146–150
Takahashi I, Matsuzaki T, Kuroki H, Hoso M (2018) Induction of osteoarthritis by injecting monosodium iodoacetate into the patellofemoral joint of an experimental rat model. PLoS One 13:e0196625
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750
Vogel R, Pal AK, Jambhrunkar S, Patel P, Thakur SS, Reategui E, Parekh HS, Saa P, Stassinopoulos A, Broom MF (2017) High-resolution single particle zeta potential characterisation of biological nanoparticles using tunable resistive pulse sensing. Sci Rep 7:17479
Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, Lorenowicz MJ (2018) Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 8:906–920
Wang HX, Kolesnikova TV, Denison C, Gygi SP, Hemler ME (2011) The C-terminal tail of tetraspanin protein CD9 contributes to its function and molecular organization. J Cell Sci 124:2702–2710
Wang K, Li F, Yuan Y, Shan L, Cui Y, Qu J, Lian F (2020) Synovial mesenchymal stem cell-derived EV-packaged miR-31 downregulates histone demethylase KDM2A to prevent knee osteoarthritis. Mol Ther Nucleic Acids 22:1078–1091
Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, Zhou J, Heng BC, Zou XH, Ouyang H, Liu H (2017) Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther 8:189
Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS, Yun YE, Han J, Lee J, Kim WS, Choi JS, Yang S, Park JH, Jo DG, Cho YW (2020) Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles 9:1735249
Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M (2011) Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A 17:1425–1436
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066
Yang YH, Lee AJ, Barabino GA (2012) Coculture-driven mesenchymal stem cell-differentiated articular chondrocyte-like cells support neocartilage development. Stem Cells Transl Med 1:843–854
Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, Yang P, Duan X, Zhang J, Fu X, Hu X, Ao Y (2020) Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater 106:328–341
Yuan X, Sun L, Jeske R, Nkosi D, York SB, Liu Y, Grant SC, Meckes DG Jr, Li Y (2022) Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J Extracell Vesicles 11:e12235
Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS (2016) Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil 24:2135–2140
Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS (2018) MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156:16–27
Zhang X, Borg EGF, Liaci AM, Vos HR, Stoorvogel W (2020) A novel three step protocol to isolate extracellular vesicles from plasma or cell culture medium with both high yield and purity. J Extracell Vesicles 9:1791450
Zhao X, Zhao Y, Sun X, Xing Y, Wang X, Yang Q (2020) Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol 8:575057
Acknowledgements
Dr. Sara Farahi and Dr. Nahid Nasiri are gratefully acknowledged for their assistance with real-time PCR and the in vivo experiments. We also appreciate Dr. Abazar Esmaeili for his help and guidance.
Funding
This study was financially supported by the Iran National Science Foundation (INSF), award number 96008901.
Author information
Authors and Affiliations
Contributions
M.H., S.H., and M.B.E. conceived and designed the experiments. M.H. carried out the experiments, wrote the manuscript, analyzed the data, and prepared Figs. 2, 3, and 4. S.H. reviewed and edited the manuscript, acquired funding, and prepared Fig. 1. A.K. performed the histological analysis and prepared Figs. 5 and 6. M.B.E. and S.H. coordinated the study and provided technical assistance. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All procedures were approved by the Animal Care and Ethics Committee at Royan Institute, Tehran, Iran (IR.ACECR.ROYAN.REC.1398.245).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseinzadeh, M., Kamali, A., Baghaban Eslaminejad, M. et al. Higher ratios of chondrocyte to mesenchymal stem cells elevate the therapeutic effects of extracellular vesicles harvested from chondrocyte/mesenchymal stem cell co-culture on osteoarthritis in a rat model. Cell Tissue Res 394, 145–162 (2023). https://doi.org/10.1007/s00441-023-03819-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03819-w