Abstract
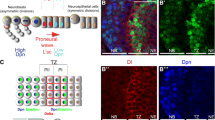
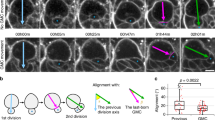
The neural stem cells of Drosophila, called neuroblasts, have the ability to self-renew and at the same time produce many different types of neurons and glial cells. In the central brain and ventral ganglia, neuroblasts are specified and delaminate from the neuroectoderm during embryonic development under the control of proneural and neurogenic genes. In contrast, in the optic lobes, neuroepithelial cells are transformed into neuroblasts postembryonically by a spatial wave of proneural gene expression. Central brain and ventral nerve cord neuroblasts manifest a short embryonic proliferation period followed by a stage of quiescence and then undergo a prolonged postembryonic proliferation period during which most of the differentiated neurons of the adult CNS are generated. While most neuroblasts belong to a type I class that produces neuronal lineages through non-self-renewing ganglion mother cells, a small subset of type II neuroblasts generates exceptionally large neuronal lineages through self-renewing intermediate progenitor cells that have a transit amplifying function. All neuroblasts in the CNS generate their neural progeny through an asymmetric cell division mode in which the interplay of apical complex and basal complex molecules in the mitotically active progenitor results in the segregation of cell fate determinants into the smaller more differentiated daughter cell. Defects in this molecular control of asymmetric cell division in neuroblasts can result in brain tumor formation. Proliferating neuroblast lineages in the developing CNS utilize transcription factor cascades as a generic mechanism for temporal patterning and birth order-dependent determination of differential neural cell fate. This contributes to the generation of a remarkable diversity of cell types in the developing CNS from a surprisingly small set of neural stem cell-like precursors.





Similar content being viewed by others
Abbreviations
- aPKC:
-
Atypical protein kinase C
- CNS:
-
Central nerve system
- Gαi:
-
G protein α i subunit 65A
- INP:
-
Intermediate neural progenitor
- Mud:
-
Mushroom body defect
- Par3:
-
Partitioning defect 3
- Par6:
-
Partitioning defect 6
- Pins:
-
Partner of Inscuteable
- Pon:
-
Partner of Numb
References
Almeida MS, Bray SJ (2005) Regulation of post-embryonic neuroblasts by Drosophila Grainyhead. Mech Dev 122:1282–1293
Artavanis-Tsakonas S, Simpson P (1991) Choosing a cell fate: a view from the Notch locus. Trends Genetics 7:403–408
Atwood SX, Prehoda KE (2009) aPKC Phosphorylates Miranda to polarize fate determinants during neuroblast asymmetric cell division. Curr Biol 19:723–729
Baumgardt M, Karlsson D, Terriente J, Diaz-Benjumea FJ, Thor S (2009) Neuronal subtype specification within a lineage by opposing temporal feed-forward loops. Cell 139:969–982
Bayraktar OA, Boone JQ, Drummond ML, Doe CQ (2010) Drosophila type II neuroblast lineages keep Prospero levels low to generate large clones that contribute to the adult brain central complex. Neural Dev 5:26
Bayraktar OA, Doe CQ (2013) Combinatorial temporal patterning in progenitors expands neural diversity. Nature 498:449–455
Beaucher M, Goodliffe J, Hersperger E, Trunova S, Frydman H, Shearn A (2007) Drosophila brain tumor metastases express both neuronal and glial cell type markers. Dev Biol 301:287–297
Bello B, Holbro N, Reichert H (2007) Polycomb group genes are required for neural stem cell survival in postembryonic neurogenesis of Drosophila. Development 134:1091–1099
Bello B, Reichert H, Hirth F (2006) The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development 133:2639–2648
Bello BC, Hirth F, Gould AP (2003) A pulse of the Drosophila Hox protein Abdominal-A schedules the end of neural proliferation via neuroblast apoptosis. Neuron 37:209–219
Bello BC, Izergina N, Caussinus E, Reichert H (2008) Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev 3:5
Benito-Sipos J, Estacio-Gomez A, Moris-Sanz M, Baumgardt M, Thor S, Diaz-Benjumea FJ (2010) A genetic cascade involving klumpfuss, nab and castor specifies the abdominal leucokinergic neurons in the Drosophila CNS. Development 137:3327–3336
Benito-Sipos J, Ulvklo C, Gabilondo H, Baumgardt M, Angel A, Torroja L, Thor S (2011) Seven up acts as a temporal factor during two different stages of neuroblast 5-6 development. Development 138:5311–5320
Berger C, Harzer H, Burkard TR, Steinmann J, van der Horst S, Laurenson AS, Novatchkova M, Reichert H, Knoblich JA (2012) FACS purification and transcriptome analysis of drosophila neural stem cells reveals a role for Klumpfuss in self-renewal. Cell Rep 2:407–418
Betschinger J, Mechtler K, Knoblich JA (2003) The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422:326–330
Betschinger J, Mechtler K, Knoblich JA (2006) Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124:1241–1253
Boone JQ, Doe CQ (2008) Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurol 68:1185–1195
Bossing T, Udolph G, Doe CQ, Technau GM (1996) The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev Biol 179:41–64
Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA (2008) The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell 14:535–546
Broadus J, Doe CQ (1995) Evolution of neuroblast identity: seven-up and prospero expression reveal homologous and divergent neuroblast fates in Drosophila and Schistocerca. Development 121:3989–3996
Brody T, Odenwald WF (2000) Programmed transformations in neuroblast gene expression during Drosophila CNS lineage development. Dev Biol 226:34–44
Buescher M, Hing FS, Chia W (2002) Formation of neuroblasts in the embryonic central nervous system of Drosophila melanogaster is controlled by SoxNeuro. Development 129:4193–4203
Campos-Ortega JA (1993) Mechanisms of early neurogenesis in Drosophila melanogaster. J Neurobiol 24:1305–1327
Caussinus E, Gonzalez C (2005) Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet 37:1125–1129
Cenci C, Gould AP (2005) Drosophila Grainyhead specifies late programmes of neural proliferation by regulating the mitotic activity and Hox-dependent apoptosis of neuroblasts. Development 132:3835–3845
Chang KC, Wang C, Wang H (2012) Balancing self-renewal and differentiation by asymmetric division: insights from brain tumor suppressors in Drosophila neural stem cells. BioEssays 34:301–310
Chell JM, Brand AH (2010) Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell 143:1161–1173
Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, van Steensel B, Micklem G, Brand AH (2006) Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev Cell 11:775–789
Colombani J, Andersen DS, Leopold P (2012) Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336:582–585
Doe CQ (1992) Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development 116:855–863
Doe CQ (2008) Neural stem cells: balancing self-renewal with differentiation. Development 135:1575–1587
Doe CQ, Technau GM (1993) Identification and cell lineage of individual neural precursors in the Drosophila CNS. Trends Neurosci 16:510–514
Egger B, Boone JQ, Stevens NR, Brand AH, Doe CQ (2007) Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Dev 2:1
Egger B, Chell JM, Brand AH (2008) Insights into neural stem cell biology from flies. Philos Trans R Soc Lond B 363:39–56
Egger B, Gold KS, Brand AH (2010) Notch regulates the switch from symmetric to asymmetric neural stem cell division in the Drosophila optic lobe. Development 137:2981–2987
Egger B, Gold KS, Brand AH (2011) Regulating the balance between symmetric and asymmetric stem cell division in the developing brain. Fly 5:237–241
Fernandez-Hernandez I, Rhiner C, Moreno E (2013) Adult neurogenesis in Drosophila. Cell Reports 3:1857–1865
Grosskortenhaus R, Pearson BJ, Marusich A, Doe CQ (2005) Regulation of temporal identity transitions in Drosophila neuroblasts. Dev Cell 8:193–202
Grosskortenhaus R, Robinson KJ, Doe CQ (2006) Pdm and Castor specify late-born motor neuron identity in the NB7-1 lineage. Genes Dev 20:2618–2627
Hartenstein V, Wodarz A (2013) Initial neurogenesis in Drosophila. Dev Biol 2:701–721
Homem CC, Knoblich JA (2012) Drosophila neuroblasts: a model for stem cell biology. Development 139:4297–4310
Ikeshima-Kataoka H, Skeath JB, Nabeshima Y, Doe CQ, Matsuzaki F (1997) Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature 390:625–629
Isshiki T, Pearson B, Holbrook S, Doe CQ (2001) Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell 106:511–521
Ito K, Hotta Y (1992) Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol 149:134–148
Izergina N, Balmer J, Bello B, Reichert H (2009) Postembryonic development of transit amplifying neuroblast lineages in the Drosophila brain. Neural Dev 4:44
Izumi Y, Ohta N, Hisata K, Raabe T, Matsuzaki F (2006) Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat Cell Biol 8:586–593
Januschke J, Gonzalez C (2008) Drosophila asymmetric division, polarity and cancer. Oncogene 27:6994–7002
Jiang Y, Reichert H (2012) Programmed cell death in type II neuroblast lineages is required for central complex development in the Drosophila brain. Neural Dev 7:3
Kambadur R, Koizumi K, Stivers C, Nagle J, Poole SJ, Odenwald WF (1998) Regulation of POU genes by castor and hunchback establishes layered compartments in the Drosophila CNS. Genes Dev 12:246–260
Karcavich R, Doe CQ (2005) Drosophila neuroblast 7-3 cell lineage: a model system for studying programmed cell death, Notch/Numb signaling, and sequential specification of ganglion mother cell identity. J Comp Neurol 481:240–251
Karcavich RE (2005) Generating neuronal diversity in the Drosophila central nervous system: a view from the ganglion mother cells. Dev Dyn 232:609–616
Karlsson D, Baumgardt M, Thor S (2010) Segment-specific neuronal subtype specification by the integration of anteroposterior and temporal cues. PLoS Biol 8:e1000368
Knoblich JA (2008) Mechanisms of asymmetric stem cell division. Cell 132:583–597
Komori H, Xiao Q, McCartney BM, Lee CY (2014) Brain tumor specifies intermediate progenitor cell identity by attenuating beta-catenin/Armadillo activity. Development 141:51–62
Kraut R, Campos-Ortega JA (1996) inscuteable, a neural precursor gene of Drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev Biol 174:65–81
Kraut R, Chia W, Jan LY, Jan YN, Knoblich JA (1996) Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature 383:50–55
Kumar A, Bello B, Reichert H (2009) Lineage-specific cell death in postembryonic brain development of Drosophila. Development 136:3433–3442
Kurusu M, Maruyama Y, Adachi Y, Okabe M, Suzuki E, Furukubo-Tokunaga K (2009) A conserved nuclear receptor, Tailless, is required for efficient proliferation and prolonged maintenance of mushroom body progenitors in the Drosophila brain. Dev Biol 326:224–236
Larsen C, Shy D, Spindler SR, Fung S, Pereanu W, Younossi-Hartenstein A, Hartenstein V (2009) Patterns of growth, axonal extension and axonal arborization of neuronal lineages in the developing Drosophila brain. Dev Biol 335:289–304
Lee CY, Andersen RO, Cabernard C, Manning L, Tran KD, Lanskey MJ, Bashirullah A, Doe CQ (2006a) Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev 20:3464–3474
Lee CY, Wilkinson BD, Siegrist SE, Wharton RP, Doe CQ (2006b) Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev Cell 10:441–449
Li L, Vaessin H (2000) Pan-neural Prospero terminates cell proliferation during Drosophila neurogenesis. Genes Dev 14:147–151
Li S, Wang C, Sandanaraj E, Aw SS, Koe CT, Wong JJ, Yu F, Ang BT, Tang C, Wang H (2014) The SCFSlimb E3 ligase complex regulates asymmetric division to inhibit neuroblast overgrowth. EMBO Rep 15:165–174
Li X, Erclik T, Bertet C, Chen Z, Voutev R, Venkatesh S, Morante J, Celik A, Desplan C (2013) Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature 498:456–462
Lin S, Lai SL, Yu HH, Chihara T, Luo L, Lee T (2010) Lineage-specific effects of Notch/Numb signaling in post-embryonic development of the Drosophila brain. Development 137:43–51
Lovick JK, Ngo KT, Omoto JJ, Wong DC, Nguyen JD, Hartenstein V (2013) Postembryonic lineages of the Drosophila brain: I. Development of the lineage associated fiber tracts Dev Biol 384:228–257
Maurange C, Cheng L, Gould AP (2008) Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell 133:891–902
Nakajima A, Isshiki T, Kaneko K, Ishihara S (2010) Robustness under functional constraint: the genetic network for temporal expression in Drosophila neurogenesis. PLoS Comput Biol 6:e1000760
Neumuller RA, Knoblich JA (2009) Wicked views on stem cell news. Nat Cell Biol 11:678–679
Neumuller RA, Richter C, Fischer A, Novatchkova M, Neumuller KG, Knoblich JA (2011) Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell 8:580–593
Novotny T, Eiselt R, Urban J (2002) Hunchback is required for the specification of the early sublineage of neuroblast 7-3 in the Drosophila central nervous system. Development 129:1027–1036
Ohshiro T, Yagami T, Zhang C, Matsuzaki F (2000) Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408:593–596
Overton PM, Meadows LA, Urban J, Russell S (2002) Evidence for differential and redundant function of the Sox genes Dichaete and SoxN during CNS development in Drosophila. Development 129:4219–4228
Pearson BJ, Doe CQ (2003) Regulation of neuroblast competence in Drosophila. Nature 425:624–628
Peng CY, Manning L, Albertson R, Doe CQ (2000) The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408:596–600
Peterson C, Carney GE, Taylor BJ, White K (2002) reaper is required for neuroblast apoptosis during Drosophila development. Development 129:1467–1476
Prokop A, Technau GM (1991) The origin of postembryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Development 111:79–88
Randhawa R, Cohen P (2005) The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab 86:84–90
Reddy BV, Rauskolb C, Irvine KD (2010) Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development 137:2397–2408
Reichert H (2011) Drosophila neural stem cells: cell cycle control of self-renewal, differentiation, and termination in brain development. Results Probl Cell Differ 53:529–546
Rhyu MS, Jan LY, Jan YN (1994) Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76:477–491
Schaefer M, Knoblich JA (2001) Protein localization during asymmetric cell division. Exp Cell Res 271:66–74
Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM (1997) The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol 189:186–204
Shen CP, Jan LY, Jan YN (1997) Miranda is required for the asymmetric localization of Prospero during mitosis in Drosophila. Cell 90:449–458
Shim J, Gururaja-Rao S, Banerjee U (2013) Nutritional regulation of stem and progenitor cells in Drosophila. Development 140:4647–4656
Siegrist SE, Haque NS, Chen CH, Hay BA, Hariharan IK (2010) Inactivation of both Foxo and reaper promotes long-term adult neurogenesis in Drosophila. Curr Biol 20:643–648
Siller KH, Cabernard C, Doe CQ (2006) The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat Cell Biol 8:594–600
Skeath JB (1999) At the nexus between pattern formation and cell-type specification: the generation of individual neuroblast fates in the Drosophila embryonic central nervous system. BioEssays 21:922–931
Skeath JB, Thor S (2003) Genetic control of Drosophila nerve cord development. Curr Opin Neurobiol 13:8–15
Sousa-Nunes R, Yee LL, Gould AP (2011) Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 471:508–512
Spana EP, Doe CQ (1996) Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17:21–26
Spana EP, Kopczynski C, Goodman CS, Doe CQ (1995) Asymmetric localization of numb autonomously determines sibling neuron identity in the Drosophila CNS. Development 121:3489–3494
Speicher S, Fischer A, Knoblich J, Carmena A (2008) The PDZ protein Canoe regulates the asymmetric division of Drosophila neuroblasts and muscle progenitors. Curr Biol 18:831–837
Suzuki T, Kaido M, Takayama R, Sato M (2013) A temporal mechanism that produces neuronal diversity in the Drosophila visual center. Dev Biol 380:12–24
Technau GM, Berger C, Urbach R (2006) Generation of cell diversity and segmental pattern in the embryonic central nervous system of Drosophila. Dev Dyn 235:861–869
Touma JJ, Weckerle FF, Cleary MD (2012) Drosophila Polycomb complexes restrict neuroblast competence to generate motoneurons. Development 139:657–666
Tran KD, Doe CQ (2008) Pdm and Castor close successive temporal identity windows in the NB3-1 lineage. Development 135:3491–3499
Truman JW, Bate M (1988) Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol 125:145–157
Truman JW, Moats W, Altman J, Marin EC, Williams DW (2010) Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster. Development 137:53–61
Tsuji T, Hasegawa E, Isshiki T (2008) Neuroblast entry into quiescence is regulated intrinsically by the combined action of spatial Hox proteins and temporal identity factors. Development 135:3859–3869
Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN (1989) numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 58:349–360
Urbach R, Technau GM (2003) Molecular markers for identified neuroblasts in the developing brain of Drosophila. Development 130:3621–3637
Urbach R, Technau GM (2004) Neuroblast formation and patterning during early brain development in Drosophila. BioEssays 26:739–751
Viktorin G, Riebli N, Popkova A, Giangrande A, Reichert H (2011) Multipotent neural stem cells generate glial cells of the central complex through transit amplifying intermediate progenitors in Drosophila brain development. Dev Biol 356:553–565
Viktorin G, Riebli N, Reichert H (2013) A multipotent transit-amplifying neuroblast lineage in the central brain gives rise to optic lobe glial cells in Drosophila. Dev Biol 379:182–194
Wang H, Cai Y, Chia W, Yang X (2006a) Drosophila homologs of mammalian TNF/TNFR-related molecules regulate segregation of Miranda/Prospero in neuroblasts. EMBO J 25:5783–5793
Wang H, Ouyang Y, Somers WG, Chia W, Lu B (2007) Polo inhibits progenitor self-renewal and regulates Numb asymmetry by phosphorylating Pon. Nature 449:96–100
Wang H, Somers GW, Bashirullah A, Heberlein U, Yu F, Chia W (2006b) Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev 20:3453–3463
Weng M, Golden KL, Lee CY (2010) dFezf/Earmuff maintains the restricted developmental potential of intermediate neural progenitors in Drosophila. Dev Cell 18:126–135
Weng M, Lee CY (2011) Keeping neural progenitor cells on a short leash during Drosophila neurogenesis. Curr Opin Neurobiol 21:36–42
Wirtz-Peitz F, Nishimura T, Knoblich JA (2008) Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell 135:161–173
Wu PS, Egger B, Brand AH (2008) Asymmetric stem cell division: lessons from Drosophila. Semin Cell Dev Biol 19:283–293
Xiao Q, Komori H, Lee CY (2012) klumpfuss distinguishes stem cells from progenitor cells during asymmetric neuroblast division. Development 139:2670–2680
Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S (2006) Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci 119:2107–2118
Yasugi S, Mizuno T (2008) Molecular analysis of endoderm regionalization. Dev Growth Differ 50(Suppl 1):S79–96
Yasugi T, Umetsu D, Murakami S, Sato M, Tabata T (2008) Drosophila optic lobe neuroblasts triggered by a wave of proneural gene expression that is negatively regulated by JAK/STAT. Development 135:1471–1480
Younossi-Hartenstein A, Nassif C, Green P, Hartenstein V (1996) Early neurogenesis of the Drosophila brain. J Comp Neurol 370:313–329
Zhong W, Chia W (2008) Neurogenesis and asymmetric cell division. Curr Opin Neurobiol 18:4–11
Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN (1996) Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron 17:43–53
Zhu S, Lin S, Kao CF, Awasaki T, Chiang AS, Lee T (2006) Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell 127:409–422
Acknowledgments
We thank Yanrui Jiang for critical reading of the manuscript. This work was supported by grants from the Swiss National Research Program 63 (4063 L 128006) and the Swiss National Science Foundation (31003A 140607) as well as by grants from the Global Research Laboratory Program (NRF-2009-00424), Brain Research Program (NRF-2009-0081465), and Stem Cell Research Program (NRF-2006-2004289) of the Korean Ministry of Science, ICT, and Future Planning (MSIP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, K.H., Reichert, H. Control of neural stem cell self-renewal and differentiation in Drosophila . Cell Tissue Res 359, 33–45 (2015). https://doi.org/10.1007/s00441-014-1914-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1914-9