Abstract
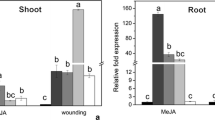
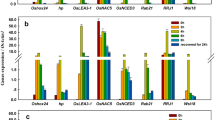
Peroxidase (POX) genes consist of a large gene family possibly contributing to self-defense, however constitutive and stress-induced expression patterns of individual gene were poorly understood in rice. We studied here the characteristic expression of two representative rice POX genes, R2329 and R2184, which are blast fungus-inducible (Sasaki et al. in Plant Cell Physiol 45:1442–1452, 2004). Basal GUS activity in R2329 promoter::GUS rice plants was 100-fold higher than that in R2184 promoter::GUS plants, and these levels reflected the transcript levels monitored by quantitative real-time RT-PCR. R2329 promoter was activated by blast fungus-infection and wounding, and R2184 promoter was activated by the fungal-infection and methyl jasmonate (MeJA)-treatment. By histochemical GUS staining analysis, constitutive R2329 and R2184 expression was commonly found in vascular bundle and exodermis in leaves and roots, while the precise expression profile was characteristic. In blast fungus inoculated R2329 promoter::GUS leaves, GUS staining was induced just around fungus-induced local lesions. Analysis of the 5′ deleted promoters suggests the presence of many kinds of stress-responsive elements in the regions between −1798 and −748 of R2329 promoter and between −1975 and −548 of R2184 promoter. These results revealed the stress-responsive characteristics of R2329 and R2184 promoters, and indicated the possible use for generation of useful transgenic plants.






Similar content being viewed by others
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Allen GC, Hall G Jr, Michalowski S, Newman W, Spiker S, Weissinger AK, Thompson WF (1996) High-level transgene expression in plant cells: effects of a strong scaffold attachment region from tobacco. Plant Cell 8:899–913
Bolwell GP, Butt VS, Davies DR, Zimmerlin A (1995) The origin of the oxidative burst in plants. Free Radic Res 23:517–532
Boter M, Ruíz-Rivero O, Abdeen A, Prat S (2004) Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev 18:1577–1591
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caruso C, Chilosi G, Leonardi L, Bertini L, Magro P, Buonocore V, Caporale C (2001) A basic peroxidase from wheat kernel with antifungal activity. Phytochemistry 58:743–750
Chu CC (1978) The N6 medium and its applications to anther culture of cereal crops. In: Proceedings of Syrup Plant Tissue Culture Peking. Science Press, pp 43–50
Collinge DB, Slusarenko AJ (1987) Plant gene expression in response to pathogens. Plant Mol Biol 9:389–410
Dean BB, Kolattukudy PE (1976) Synthesis of suberin during wound-healing in jade leaves, tomato fruit, and bean pods. Plant Physiol 58:411–416
Elmayan T, Tepfer M (1995) Evaluation in tobacco of the organ specificity and strength of the rolD promoter, domain A of the 35S promoter and the 35S2 promoter. Transgenic Res 4:388–396
Evrard A, Meynard D, Guiderdoni E, Joudrier P, Gautier MF (2007) The promoter of the wheat puroindoline-a gene (PinA) exhibits a more complex pattern of activity than that of the PinB gene and is induced by wounding and pathogen attack in rice. Planta 225:287–300
Goodman RN, Novacky AJ (1994) The hypersensitive reaction in plants to pathogens. A resistance phenomenon. APS Press, St. Paul
Guiderdoni E, Cordero MJ, Vignols F, Garcia-Garrido JM, Lescot M, Tharreau D, Meynard D, Ferriere N, Notteghem JL, Delseny M (2002) Inducibility by pathogen attack and developmental regulation of the rice Ltp1 gene. Plant Mol Biol 49:683–699
Hattori T, Terada T, Hamasuna S (1995) Regulation of the Osem gene by abscisic acid and the transcriptional activator VP1: analysis of cis-acting promoter elements required for regulation by abscisic acid and VP1. Plant J 7:913–925
Hiei Y, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of boundaries of the T-DNA. Plant J 6:271–282
Hilaire E, Young SA, Willard LH, McGee JD, Sweat T, Chittor JM, Guikema JA, Leach JE (2001) Vascular defense response in rice; peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Mol Plant Microbe Interact 14:1411–1419
Hiraga S, Ito H, Yamakawa H, Ohtsubo N, Seo S, Mitsuhara I, Matsui H, Honma M, Ohashi Y (2000) An HR-induced tobacco peroxidase gene is responsive to spermine, but not to salicylate, metyl jasmonate, and ethephon. Mol Plant Microbe Interact 13:210–216
Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42:462–468
Holsters M, de Waele D, Depicker A, Messens E, van Montagu M, Schell J (1978) Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet 163:182–187
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplastic barrier. J Exp Bot 52:2245–2264
Huang N, Sutliff TD, Litts JC, Rodriguez RL (1990) Classification and characterization of the rice alpha-amylase multigene family. Plant Mol Biol 14:655–668
Ito H, Hiraga S, Tsugawa H, Matsui H, Honma M, Otsuki Y, Murakami T, Ohashi Y (2000) Xylem-specific expression of wound-inducible rice peroxidase genes in transgenic plants. Plant Sci 155:85–100
Itzhaki H, Maxson JM, Woodson WR (1994) An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST) gene. Proc Natl Acad Sci USA 91:8925–8929
Kawaoka A, Matsunaga E, Endo S, Kondo S, Yoshida K, Shinmyo A, Ebinuma H (2003) Ectopic expression of a horseradish peroxidase enhances growth rate and increases oxidative stress resistance in hybrid aspen. Plant Physiol 132:1177–1185
Kosugi S, Ohashi Y, Nakajima K, Arai Y (1990) An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci 70:133–140
Kristensen BK, Bloch H, Rasmussen SK (1999) Barley coleoptile peroxidases. Purification, molecular cloning, and induction by pathogens. Plant Physiol 120:501–512
Lagrimini LM, Joly RJ, Dunlap JR, Liu TTY (1997) The consequence of peroxidase overexpression in transgenic plants on root growth and development. Plant Mol Biol 33:887–895
Menke FL, Champion A, Kijne JW, Memelink J (1999) A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO J 18:4455–4463
Monke G, Altschmied L, Tewes A, Reidt W, Mock HP, Baumlein H, Conrad U (2004) Seed-specific transcription factors ABI3 and FUS3: molecular interaction with DNA. Planta 219:158–166
Moreno AB, Penas G, Rufat M, Bravo JM, Estopa M, Messeguer J, San Segundo B (2005) Pathogen-induced production of the antifungal AFP protein from Aspergillus giganteus confers resistance to the blast fungus Magnaporthe grisea in transgenic rice. Mol Plant Microbe Interact 18:960–972
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nishiuchi T, Shinshi H, Suzuki K (2004) Rapid and transient activation of transcription of the ERF3 gene by wounding in tobacco leaves: possible involvement of NtWRKYs and autorepression. J Biol Chem 279:55355–55361
Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15:1591–1604
Ohta S, Mita S, Hattori T, Nakamura K (1990) Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31:805–813
Park HC, Kim ML, Kang YH, Jeon JM, Yoo JH, Kim MC, Park CY, Jeong JC, Moon BC, Lee JH, Yoon HW, Lee SH, Chung WS, Lim CO, Lee SY, Hong JC, Cho MJ (2004) Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol 135:2150–2161
Passardi F, Longet D, Penel C, Dunand C (2004a) The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65:1879–1893
Passardi F, Penel C, Dunand C (2004b) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9:534–540
Quiroga M, Guerrero C, Botella MA, Barceló A, Amaya I, Medina MI, Alonso FJ, de Forchetti SM, Tigier H, Valpuesta V (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol 122:1119–1127
Sasaki K, Iwai T, Hiraga S, Kuroda K, Seo S, Mitsuhara I, Miyasaka A, Iwano M, Ito H, Matsui H, Ohashi Y (2004) Ten rice peroxidases redundantly respond to multiple stresses including infection with rice blast fungus. Plant Cell Physiol 45:1442–1452
Schweizer P, Buchala A, Silverman P, Seskar M, Raskin I, Metraux JP (1997) Jasmonate-inducible genes are activated in rice by pathogen attack without a concomitant increase in endogenous jasmonic acid levels. Plant Physiol 114:79–88
Sesma A, Osbourn AE (2004) The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431:582–586
Showalter AM (1993) Structure and function of plant cell wall proteins. Plant Cell 5:9–23
Slatter RE, Dupree P, Gray JC (1991) A scaffold-associated DNA region is located downstream of the pea plastocyanin gene. Plant Cell 3:1239–1250
Stoessl A (1967) The antifungal factors in barley. IV. Isolation, structure, and synthesis of the hordatines. Can J Chem 45:1745–1760
Strompen G, Gruner R, Pfitzner UM (1998) An as-1-like motif controls the level of expression of the gene for the pathogenesis-related protein 1a from tobacco. Plant Mol Biol 37:871–883
Takeda S, Sugimoto K, Otsuki H, Hirochika H (1999) A 13-bp cis-regulatory element in the LTR promoter of the tobacco retrotransposon Tto1 is involved in responsiveness to tissue culture, wounding, methyl jasmonate and fungal elicitors. Plant J 18:383–393
Thordal-Christensen H, Brandt J, Cho BH, Rasmussen SK, Gregersen PL, Smedegaard-Petersen V, Collinge DB (1992) cDNA cloning and characterization of two barley peroxidases transcripts induced differentially by the powdery mildrew fungus Erysiphegraminis. Physiol Mol Plant Pathol 40:395–409
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47:969–976
van Loon LC, Pierpoint WS, Boller T, Conejero V (1994) Recommendation for naming plant pathogenesis-related proteins. Plant Mol Biol Rep 12:245–264
Vera P, Torneo P, Conejero V (1993) Cloning and expression analysis of a viroid-induced peroxidase from tomato plants. Mol Plant Microbe Interact 6:790–794
Welinder KG, Justesen AF, Kjaersgard IV, Jensen RB, Rasmussen SK, Jespersen HM, Duroux L (2002) Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. Eur J Biochem 269:6063–6081
Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322:681–692
Yamada M, Kiyosawa S, Yamaguchi T, Hirano T, Kobayashi T, Kushibuti K, Watanabe S (1976) Proposal of a new method for differentiating races of Pyricularia orizae cavara in Japan. Ann Phytopath Soc Jpn 42:216–219
Young SA, Guo A, Guikema JA, White FF, Leach JE (1995) Rice cationic peroxidase accumulates in xylem vessels during incompatible interactions with Xanthomonas oryzae pv. oryzaae. Plant Physiol 107:1333–1341
Acknowledgment
We thank Dr. T. Imbe of the National Institute of Crop Science (NICS) for providing IL-7 rice seed, A. Miyasaka of NICS for providing M. grisea race 003, and Prof. K. Nakamura of Nagoya University for providing the pIG121-Hm vector. We acknowledge Y. Naito, R. Takabatake, S. Katou and K. Gomi for their helpful advice regarding the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Perez-Martin.
The authors Katsutomo Sasaki and Ohtsu Yuichi are equally contribute to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sasaki, K., Yuichi, O., Hiraga, S. et al. Characterization of two rice peroxidase promoters that respond to blast fungus-infection. Mol Genet Genomics 278, 709–722 (2007). https://doi.org/10.1007/s00438-007-0286-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-007-0286-1