Abstract
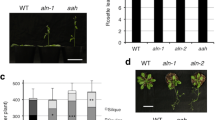
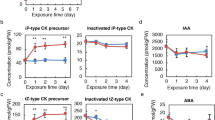
A phenotypic screen was employed to isolate Arabidopsis plants that are deficient in their ability to utilize or sense acetate. The screening strategy, based on resistance to the toxic acetate analogue monofluoroacetic acid, was adapted from one that has been used successfully to identify important metabolic and regulatory genes involved in acetate metabolism in fungi. Following conventions established from the fungal work, the mutants were called acn mutants for ac etate n on-utilization. Three highly resistant plant lines were the focus of genetic and physiological studies. Mutant acn1 appears to be a true acetate non-utilizing mutant, as it displays increased sensitivity to exogenous acetate. The progeny of the original acn2 mutant did not germinate, even in the presence of sucrose as an exogenous carbon source. The germination of seeds from the F3 generation depended on the sucrose concentration in the medium. Only a small proportion of seeds germinated in the absence of exogenous sucrose and in the presence of 100 mM sucrose, but up to 70% of seeds germinated on 20 mM sucrose. Mutant acn3 exhibited sensitivity to exogenous sucrose, showing significant chlorosis on medium containing 20 mM sucrose, but no chlorosis when grown in the absence of exogenous sucrose. This phenotype was alleviated if acetate was provided. The acn mutants demonstrate that disrupting organic acid utilization can have profound affects on carbohydrate metabolism.






Similar content being viewed by others
References
Apirion D (1965) The two-way selection of mutants and revertants in respect of acetate utilization and resistance to fluoroacetate in Aspergillus nidulans. Genet Res 6:317–329
Armitt S, McCullough W, Roberts CR (1976) Analysis of acetate non-utilizing (acu) mutants in Aspergillus nidulans. J Gen Microbiol 92:263–282
Canvin DT, Beevers H (1961) Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem 236:988–995
Coruzzi GM, Zhou L (2001) Carbon and nitrogen sensing and signaling in plants: emerging ‘matrix effects’. Curr Opin Plant Biol 4:247–253.
Cozzone AJ (1998) Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annu Rev Microbiol 52:127–164.
Eastmond PJ, Germain V, Lange PR, Bryce JH, Smith SM, Graham IA (2000) Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc Natl Acad Sci USA 97:5669–5674
Feldmann KA, Malmberg RL, Dean C (1994) Mutagenesis in Arabidopsis. In: Meyerowitz EM, Somerville CR (eds) Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 137–172
Flavell RB, Fincham JRS (1968) Acetate non-utilizing mutants of Neuospora crassa. 1. Mutant isolation, complementation studies and linkage relationships. J Bacteriol 95:1056–1062
Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham IA, Baker A, Holdsworth M (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21:2912–2922
Ho S-L, Chao Y-C, Tong W-F, Yu SM (2001) Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol 125:877–890
Hooks MA (2002). Molecular biology, enzymology, and physiology of β-oxidation. In: Baker A, Graham IA (eds) Plant peroxisomes. Kluwer Academic, Dordrecht, pp 19–55
Katz ME, Hynes MJ (1989) Isolation and analysis of the acetate regulatory gene, facB, from Aspergillus nidulans. Mol Cell Biol 9:5696–5701
Kindle KL (1987) Expression of a gene for a light-harvesting chlorophyll- a -binding chlorophyll- b -binding protein in Chlamydomonas-reinhardtii: effect of light and acetate. Plant Mol Biol 9:547–563
Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47:509–540
Lauble H, Kennedy MC, Emptage MH, Beinert H, Stout CD (1996) The reaction of fluorocitrate with aconitase and the crystal structure of the enzyme-inhibitor complex. Proc Natl Acad Sci USA 93:13699–13703
McCammon MT (1996) Mutants of Saccharomyces cerevisiae with defects in acetate metabolism: isolation and characterisation of Acn- mutants. Genetics 144:57–69
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–496
Oh M-K, Rohlin L, Kao KC, Liao JC (2002) Global expression profiling of acetate-grown Escherichia coli. J Biol Chem 277:13175–13183
Oliveira IC, Coruzzi GM (1999) Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiol 121:301–309
Owen NE, Chaure PT, Connerton IF (1992) Isolation and characterization of new fluoroacetate resistant/acetate non-utilizing mutants of Neurospora crassa. J Gen Microbiol 138:2599–2608
Page DR, Grossniklaus U (2002) The art and design of genetic screens: Arabidopsis thaliana. Nat Rev Genet 3:124–136
Peters RA, Wakelin RW, Buffa P, Thomas LC (1953) Biochemistry of fluoroacetate poisoning: the isolation and some properties of the fluorotricarboxylic acid inhibitor of citrate metabolism. Proc R Soc London Ser B 140:497–507
Russel L, Larner V, Kurup S, Bougourd S, Holdsworth MJ (2000) The Arabidopsis COMATOSE locus regulates germination potential. Development 127:3759–3767
Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2:1027–1038
Shockey JM, Fulda MS, Browse JA (2003) Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme A synthetases. Plant Physiol 129:1710–1722
Todd RB, Murphy RL, Martin HM, Sharp JA, Davis, MA, Katz ME, Hynes MJ (1997a) The acetate regulatory gene facB of Aspergillus nidulans encodes a Zn(II)2Cys6 transcriptional activator. Mol Gen Genet 254:495–504
Todd RB, Kelly JM, Davis MA, Hynes MJ (1997b) Molecular characterization of mutants of the acetate regulatory gene facB of Aspergillus nidulans. Fungal Genet Biol 22:92–102
Vanlerberghe GC, McIntosh L (1996) Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol 111:589–595
Wintermans JFGM, de Mots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta 109:448–453
Acknowledgements
We wish to thank Dr. Michael Holdsworth and Dr. Steven Footit for providing the cts1 mutant for our initial studies. This research was supported by a grant from the British Biotechnology and Biological Sciences Research Council (5/P14659) and postgraduate student funding from the University of Wales at Bangor. All work was carried out in compliance with United Kingdom and local laws governing genetic experimentation
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Jürgens
Rights and permissions
About this article
Cite this article
Hooks, M.A., Turner, J.E., Murphy, E.C. et al. Acetate non-utilizing mutants of Arabidopsis: evidence that organic acids influence carbohydrate perception in germinating seedlings. Mol Genet Genomics 271, 249–256 (2004). https://doi.org/10.1007/s00438-004-0985-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-004-0985-9