Abstract
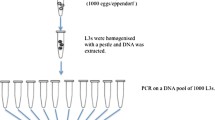
This study was conducted to determine single nucleotide polymorphisms (SNPs) and the benzimidazole (BZ) resistance in strongyle nematode egg populations in horses using molecular techniques. A total of 200 fecal samples were collected from horses in 26 farms in two provinces (Kayseri and Nevşehir) of the Central Anatolia Region of Türkiye between May and August 2022. The flotation method was used to detect strongyle nematode eggs in the fecal samples of the horses. Afterward, strongyle nematode eggs were collected, and the allele-specific polymerase chain reaction (AS-PCR) technique was used to detect the BZ resistance. BZ-susceptible and BZ-resistant PCR products were sequenced to determine single nucleotide polymorphisms (SNPs) in the β-tubulin isotype 1 gene. The strongyle nematode eggs were determined in 85 (42.5%) out of 200 fecal samples. AS-PCR detected 50.58% (43/85) BZ-resistant (homozygous resistant) and 36.4% (31/85) BZ-susceptible (homozygous susceptible) genes in the strongyle eggs. Both BZ-resistant and BZ-susceptible genes (heterozygous) were determined in 11 samples. BZ-resistant and BZ-susceptible allele frequencies were determined as 57.0% (48.5/85) and 43.0% (36.5/85), respectively. SNPs were detected only in codon 200 of the β-tubulin isotype 1 gene in four sequenced isolates of the two resistant and two susceptible isolates. This study is the first molecular report on BZ resistance in strongyle nematode eggs in horses in Türkiye. The widespread prevalence of BZ-resistant alleles in equine strongyle nematodes shows the requirement for the immediate usage of other anthelmintics instead of the BZ group drugs for the effective management and control of equine strongyle nematodes.


Similar content being viewed by others
Data availability
All data is involved in the current paper.
References
Arslan MO, Umur S (1998) The helminth and Eimeria (Protozoa) species in horse and donkey in Kars province of Turkey. Turkish J Parasitol 22:180–184
Aypak S (2013) A helmintological investigation in the different breeding horses. Animal Health Prod Hyg 2:152–155
Blackhall WJ, Kuzmina T, von Samson-Himmelstjerna G (2011) β-Tubulin genotypes in six species of cyathostomins from anthelmintic-naive Przewalski and benzimidazole-resistant brood horses in Ukraine. Parasitol Res 109(4):1199–1203
Bull KE, Allen KJ, Hodgkinson JE, Peachey LE (2023) The first report of macrocyclic lactone resistant cyathostomins in the UK. Int J Parasitol: Drugs Drug Resist 21:125–130
Ceylan O, Dik B, Ceylan C, Semassel A, Deri̇nbay Eki̇ci̇ O, Sönmez G (2020) Gastrointestinal helminths detected in wild horses in Konya Province, Turkey. Turk J Vet Anim Sci 44:662–667
Cirak VY, Girisgin AO (2021) Parasites of horses, donkeys and mules in Turkey. Turk Parazitol Derg 45:56–75
Cirak VY, Gulegen E, Bauer C (2004) Benzimidazole resistance in cyathostomin populations on horse farms in western Anatolia, Turkey. Parasitol Res 93:392–395
Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, Waller PJ (1992) World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 44:35–44
Coles GC, Jackson F, Pomroy WE, Prichard RK, von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J (2006) The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 136:167–185
Collobert-Laugier C, Hoste H, Sevin C, Dorchies P (2002) Prevalence, abundance and site distribution of equine small strongyles in Normandy, France. Vet Parasitol 110:77–83
Dărăbuş G, Badea C, Oprescu I, Morariu S, Mederle N, Ilie M, Suici T, Imre M (2021) Survey of anthelmintic resistance in a Romanian horse stud using three different methods. Pol J Vet Sci 24(1):145–149
Duszynski DW, Wilber PG (1997) A guideline for the preparation of species descriptions in the Eimeriidae. J Parasitol 83(2):333–336
Elard L, Comes AM, Humbert JF (1996) Sequences of beta-tubulin cDNA from benzimidazole-susceptible and -resistant strains of Teladorsagia circumcincta, a nematode parasite of small ruminants. Mol Biochem Parasitol 79:249–253
Elard L, Sauve C, Humbert JF (1998) Fitness of benzimidazole-resistant and -susceptible worms of Teladorsagia circumcincta, a nematode parasite of small ruminants. Parasitology 117:571–578
Erez MS, Kozan E (2018) Anthelmintic resistance in farm animals. Kocatepe Vet J 11(3):322–330
Gajadhar AA (1994) Host specificity studies and oocyst description of a Cryptosporidium sp. isolated from ostriches. Parasitol Res 80:316–319
Geary TG, Hosking BC, Skuce PJ, von Samson-Himmelstjerna G, Maeder S, Holdsworth P, Pomroy W, Vercruysse J (2012) World association for the advancement of veterinary parasitology (W.A.A.V.P.) guideline: anthelmintic combination products targeting nematode infections of ruminants and horses. Vet Parasitol 190:306–316
Gokbulut C, McKellar QA (2018) Anthelmintic drugs used in equine species. Vet Parasitol 261:27–52
Gul A, Deger S, Ayaz E (2003) The prevalence of helminth species according to faecal examination in equids in different cities in Turkey 6. Turkish J Vet Anim Sci 27:195–199
Gurler AT, Bolukbas CS, Acici M, Umur S (2010) Check list of the helminths of equines in Turkey. Turkish J Parasitol 34:40–44
Hinney B, Wirtherle NC, Kyule M, Miethe N, Zessin KH, Clausen PH (2011) A questionnaire survey on helminth control on horse farms in Brandenburg, Germany and the assessment of risks caused by different kinds of management. Parasitol Res 109:1625–1635
Hodgkinson JE, Clark HJ, Kaplan RM, Lake SL, Matthews JB (2008) The role of polymorphisms at beta tubulin isotype 1 codons 167 and 200 in benzimidazole resistance in cyathostomins. Int J Parasitol 38:1149–1160
Humbert JF, Cabaret J, Elard L, Leignel V, Silvestre A (2001) Molecular approaches to studying benzimidazole resistance in trichostrongylid nematode parasites of small ruminants. Vet Parasitol 101:405–414
Jürgenschellert L, Krücken J, Bousquet E, Bartz J, Heyer N, Nielsen MK, von Samson-Himmelstjerna G (2022) Occurrence of strongylid nematode parasites on horse farms in Berlin and Brandenburg, Germany, with high seroprevalence of Strongylus vulgaris infection. Front Vet Sci 9:892920
Kaplan RM, Klei TR, Lyons ET, Lester G, Courtney CH, French DD, Tolliver SC, Vidyashankar AN, Zhao Y (2004) Prevalence of anthelmintic resistant cyathostomes on horse farms. J Am Vet Med Assoc 225:903–910
Karaca M, Ayaz E, Tütüncü M, Gül A, Akkan HA (2005) Distribution of helmint infections and some blood parameters in horses living in the region of Van. Van Vet J 16:71–74
Kozan E, Guzel H (2015) Helminths found by faecal examination in the equine in Afyonkarahisar region. Kocatepe Vet J 8(2):18–21
Kumar S, Garg R, Kumar S, Banerjee PS, Ram H, Prasad A (2016) Benzimidazole resistance in equine cyathostomins in India. Vet Parasitol 218:93–97
Kwa MS, Veenstra JG, Van Dijk M, Roos MH (1995) Beta-tubulin genes from the parasitic nematode Haemonchus contortus modulate drug resistance in Caenorhabditis elegans. J Mol Biol 246:500–510
Lake SL, Matthews JB, Kaplan RM, Hodgkinson JE (2009) Determination of genomic DNA sequences for beta-tubulin isotype 1 from multiple species of cyathostomin and detection of resistance alleles in third-stage larvae from horses with naturally acquired infections. Parasit Vectors 2(Suppl 2):S6
Lichtenfels JR, Kharchenko VA, Dvojnos GM (2008) Illustrated identification keys to strongylid parasites (Strongylidae: Nematoda) of horses, zebras and asses (Equidae). Vet Parasitol 156:4–161
Mangassa B, Tafese W (2016) Prevalence of strongyle infection and associated risk factors in horse and donkeys in and around Batu Town, Eastshoa, Oromia Regional State, Ethiopia. Food Sci Qual Manag 54:66–71
Mathewos M, Fesseha H, Yirgalem M (2021) Study on strongyle infection of donkeys and horses in Hosaena District, Southern Ethiopia. Vet Med (Auckl) 12:67–73
Mohanraj K, Subhadra S, Kalyanasundaram A, Ilangopathy M, Raman M (2017) Genotyping of benzimidazole resistant and susceptible isolates of Haemonchus contortus from sheep by allele specific PCR. J Parasit Dis 41:282–288
Morariu S, Mederle N, Badea C, Dărăbuş G, Ferrari N, Genchi C (2016) The prevalence, abundance and distribution of cyathostomins (small stongyles) in horses from Western Romania. Vet Parasitol 223:205–209
Pape M, Posedi J, Failing K, Schnieder T, von Samson-Himmelstjerna G (2003) Analysis of the beta-tubulin codon 200 genotype distribution in a benzimidazole-susceptible and -resistant cyathostome population. Parasitology 127:53–59
Peregrine AS, Molento MB, Kaplan RM, Nielsen MK (2014) Anthelmintic resistance in important parasites of horses: does it really matter? Vet Parasitol 201:1–8
Pierce BA (2003) Genetics, a conceptual approach. W.H. Freeman and Company, NY, USA, pp 671–672
Rehbein S, Visser M, Winter R (2013) Prevalence, intensity and seasonality of gastrointestinal parasites in abattoir horses in Germany. Parasitol Res 112:407–413
Romero C, Heredia R, Miranda L, Arredondo M (2020) Prevalence of gastrointestinal parasites in horses of Central Mexico. Open J Vet Med 10:117–125
Roos MH, Kwa MSG, Grant WN (1995) New genetic and practical implications of selection for anthelmintic resistance in parasitic nematodes. Parasitol Today 11:148–150
Rufener L, Mäser P, Roditi I, Kaminsky R (2009) Haemonchus contortus acetylcholine receptors of the DEG-3 subfamily and their role in sensitivity to monepantel. PLoS Pathog 5:e1000380
Saeed MA, Beveridge I, Abbas G, Beasley A, Bauquier J, Wilkes E, Jacobson C, Hughes KJ, El-Hage C, O’Handley R, Hurley J, Cudmore L, Carrigan P, Walter L, Tennent-Brown B, Nielsen MK, Jabbar A (2019) Systematic review of gastrointestinal nematodes of horses from Australia. Parasit Vectors 12:188
Seyoum Z, Zewdu A, Dagnachew S, Bogale B (2017) Anthelmintic resistance of strongyle nematodes to ivermectin and fenbendazole on cart horses in Gondar, Northwest Ethiopia. Biomed Res Int 5163968
Silvestre A, Cabaret J (2002) Mutation in position 167 of isotype 1 beta-tubulin gene of Trichostrongylid nematodes: role in benzimidazole resistance? Mol Biochem Parasitol 120:297–300
Silvestre A, Humbert JF (2000) A molecular tool for species identification and benzimidazole resistance diagnosis in larval communities of small ruminant parasites. Exp Parasitol 95:271–276
Tiwari J, Kolte AP, Kumar S, Swarnkar CP, Singh D, Pathak KML (2006) Diagnosis of benzimidazole resistance in Haemonchus contortus of sheep by allele specific PCR. Asian Australas J Anim Sci 20:7–11
Tiwari J, Kumar S, Kolte AP, Swarnkar CP, Singh D, Pathak KML (2006) Detection of benzimidazole resistance in Haemonchus contortus using RFLP-PCR technique. Vet Parasitol 138:301–307
Toktamıs G, Yaman M (2012) The distribution of gastrointestinal helminths in thoroughbred race horses. Van Vet J 23(1):35–39
Umur S, Acici M (2009) A survey on helminth infections of equines in the Central Black Sea region, Turkey. Turk J Vet Anim Sci 33(5):373–378
Uslu U, Guçlu F (2007) Prevalence of endoparasites in horses and donkeys in Turkey. Bull Vet Inst Pulawy 51:237–240
Vera JHS, Fachiolli DF, Ramires LM, de Lima Saes I, Yamada PH, Gonçalves JA, de Oliveira K, do Amarante AFT, de Soutello RVG (2020) Efficacy of ivermectin, moxidectin and fenbendazole in equine in Brazil. Vet Parasitol Reg Stud Rep 20:00374
von Samson-Himmelstjerna G, Harder A, Pape M, Schnieder T (2001) Novel small strongyle (Cyathostominae) beta-tubulin sequences. Parasitol Res 87:122–125
von Samson-Himmelstjerna G, Pape M, von Witzendorff C, Schnieder T (2002) Allele-specific PCR for the beta-tubulin codon 200 TTC/TAC polymorphism using single adult and larval small strongyle (Cyathostominae) stages. J Parasitol 88:254–257
von Samson-Himmelstjerna G, von Witzendorff C, Sievers G, Schnieder T (2002) Comparative use of faecal egg count reduction test, egg hatch assay and beta-tubulin codon 200 genotyping in small strongyles (cyathostominae) before and after benzimidazole treatment. Vet Parasitol 108:227–235
von Samson-Himmelstjerna G et al (2003) TaqMan minor groove binder real-time PCR analysis of beta-tubulin codon 200 polymorphism in small strongyles (Cyathostomin) indicates that the TAC allele is only moderately selected in benzimidazole-resistant populations. Parasitology 127:489–496
von Samson-Himmelstjerna G, Fritzen B, Demeler J, Schürmann S, Rohn K, Schnieder T, Epe C (2007) Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet Parasitol 144:74–80
von Samson-Himmelstjerna G et al (2009) Standardization of the egg hatch test for the detection of benzimidazole resistance in parasitic nematodes. Parasitol Res 105:825–834
Winterrowd CA, Pomroy WE, Sangster NC, Johnson SS, Geary TG (2003) Benzimidazole-resistant beta-tubulin alleles in a population of parasitic nematodes (Cooperia oncophora) of cattle. Vet Parasitol 117:161–172
Acknowledgements
We would like to thank Prof. Eugene Steel (Erciyes University Proofreading & Editing Office) for proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The sample collection was performed by Zuhal Onder, Arif Ciloglu, Gamze Yetismis, and Faruk Karabulut. All the authors contributed substantially to molecular analysis. The first draft of the manuscript was written by Zuhal Onder. Alparslan Yildirim, Abdullah Inci, and Onder Duzlu revised the manuscript, and all the authors mentioned in the manuscript contributed to the revision of the manuscript to the present form. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Abdul Jabbar
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Onder, Z., Yildirim, A., Duzlu, O. et al. Detection of SNPs and benzimidazole resistance in strongyle nematode eggs of horses by allele-specific PCR. Parasitol Res 122, 2037–2043 (2023). https://doi.org/10.1007/s00436-023-07903-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07903-6