Abstract
Because the number of wild raccoons in Germany is increasing constantly, it appears to be economic reasonable to use their meat as food. For this purpose, it is essential to generate data regarding the pathogen load of the meat to be consumed and handled. It is known that raccoons, particularly in Germany, show a high seroprevalence of Toxoplasma gondii. Because serological data only indicates contact of a host to a parasite additional direct detection is needed to prove presence of parasitic stages in particular tissues. Therefore, a total of 150 samples from raccoons with known serostatus were tested and quantified using magnetic-capture real-time PCR for Toxoplasma gondii. As it represents potentially consumption-relevant parts of raccoons, meat from forelimb and hindlimb was examined. Samples were stratified into three groups based on the animals’ serostatus (each 50 negative, low positive, and high positive). All samples from seronegative animals were found negative by MC-PCR as well. In a total of 56 meat samples from 100 seropositive animals, T. gondii DNA was detected. Statistically significant more samples were positive by MC-PCR in the high positive than in the low positive serostatus group (38/50 vs. 18/50, p < 0.0001). Furthermore, samples from the former group were also found to have statistically significant higher DNA equivalent values compared to samples from the low positive serostatus group (p < 0.0001). These results suggest that meat from seropositive raccoons may contain considerable numbers of T. gondii presenting a potential public health risk for humans whilst handling and consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii is a coccidian parasite, which can probably infect all warm-blooded animals as intermediate hosts and cats as definitive hosts (Tenter et al. 2000). Whilst only Felidae excrete oocysts, all warm-blooded animals can accommodate tissue cysts in brain or muscles (Dubey 2022). Humans can become infected by a wide range of exposures. Tissue cysts containing infectious bradyzoites can be taken up through contaminated raw or undercooked meat of food-producing animals or wildlife (Jones and Dubey 2012). Additionally, the infectious oocysts can be transmitted through ingestion of contaminated food, soil, or water by cat faeces (Jones et al. 2014). Toxoplasmosis usually stays subclinical or is accompanied by mild symptoms of illness like fever, malaise, and lymphadenopathy. However, in people with pre-existing disease or immunosuppression, severe disease processes with neurologic involvement, most commonly due to encephalitis, and up to mortality may occur (Jones et al. 2014). Primary maternal infection can lead to transmission of the parasite to the foetus and a wide range of clinical manifestations like ocular disease, hydrocephalus, and intracerebral calcifications, or even abortion may arise (Dubey 2022). Due to its medical and veterinary importance, T. gondii is considered one of the most well-studied parasites (Dubey 2022). However, many studies on food-producing animals and game are mainly focusing on the presence of antibodies (Berger-Schoch et al. 2011; Bier et al. 2020; Lundén et al. 2002; Račka et al. 2015). Furthermore, economic and public health aspects regarding wild canids and other carnivores like raccoons, which are mainly raised for fur or meat production, have also been discussed recently (Dubey et al. 2021).
The raccoon (Procyon lotor) originated in North America and became established in Europe at the beginning of the past century (Kauhala 1996; Lutz 1995). As the spread increased strongly from the 1970s onwards (Lutz 1995), population management measures have become more and more important since this uncontrolled distribution (Beltrán-Beck et al. 2012; Salgado 2018). Along to this, the German Hunting Association (DJV) has recorded a strong increase in the annual hunting bag data, from about 8000 in the year 1999/2000 to more than 200,000 in 2019/2020 (German Hunting Association 2021). With the increasing popularity of eating game (German Hunting Association 2019), the use of raccoon meat may also be increasingly conceivable, as well as in other countries such as the USA, even though raccoon is still consumed less than the usual game species there (Burger 2000; Gaines et al. 2000; German Hunting Association 2020; Goguen and Riley 2020). However, raccoon meat consumption is rather rare in Germany, but is considered to be a delicacy amongst hunters (German Hunting Association 2020).
Using serological analyses, a large number of individuals can be examined at low cost, so these are preferred for screening (Opsteegh et al. 2011). A positive serological result only indicates exposure to the parasite, but does not confirm presence of the parasite in host tissues (Dubey 2022). However, the presence of antibodies may indicate an infection risk by consuming meat, if there is a strong correlations between the presence of antibodies against T. gondii and tissue cysts (Opsteegh et al. 2011). This correlation can be different in the various host species. Whilst strong correlations were found for pigs and sheep (Dubey et al. 2008; Gamble et al. 2005; Opsteegh et al. 2010), this was not the case for cattle (Opsteegh et al. 2011). Therefore, it is needed to examine such correlation for a specific species, i.e. the raccoon. Additionally, different parts of the organism may be affected to varying degrees by tissue cysts (Dubey 2022; Koethe et al. 2015; Tenter et al. 2000). Thus, to assess the human health risk, T. gondii infestation of consumption-relevant meat parts from raccoons needs to be evaluated.
Recent studies demonstrated a high seroprevalence of T. gondii in German raccoons (Engel et al. 2022; Heddergott et al. 2017; Heddergott and Müller 2020). However, seropositive animals do not immediately pose a risk to humans, as they do not always harbour tissue cysts with infectious parasites (Halos et al. 2010). Nevertheless, when performed conventionally, essential direct detection is laborious, costly, and insufficiently sensitive (Algaba et al. 2017). Due to this, there is not much published information on PCR for T. gondii in rarely consumed species like raccoons. For naturally infected feral raccoons, one study was conducted in Poland using a conventional PCR to analyse brain, lung, and heart of the animals for T. gondii DNA (Kornacka et al. 2018). However, conventional DNA extraction methods are not very sensitive as only small amounts of tissue with a maximum of 50 mg can typically be examined and the distribution of T. gondii tissue cysts is non-homogeneous in hosts (Opsteegh et al. 2010). Due to a lack of sensitivity, Opsteegh et al. (2010) developed a method in which up to 100 g of tissue can be analysed for the presence of T. gondii DNA, by sequence-specific DNA extraction using magnetic capture followed by real-time PCR. Since then, this magnetic-capture PCR (MC-PCR) has been successfully applied to various animal species or animal products with several minor modifications and different experimental setups (Gomez-Samblas et al. 2015; Hosein et al. 2016; Juránková et al. 2013; Koethe et al. 2015; Nicholas et al. 2018; Stollberg et al. 2021). Additionally, real-time quantitative PCR (qPCR) offers the advantage for quantification of parasite DNA. In general, PCR methods itself are sensitive, as they can detect DNA from as few as one tachyzoite, specific, and enable quick diagnosis (Dubey 2022). Since there are different types of PCR, quantitative MC-PCR was found to be the most sensitive method for detecting T. gondii DNA (Stollberg et al. 2021). Because of the high seroprevalence of German raccoons (Engel et al. 2022; Heddergott et al. 2017; Heddergott and Müller 2020), this study focuses on direct detection of T. gondii DNA in consumption-relevant raccoon meat by MC-PCR to provide more relevant information for future human health risk evaluation. To the authors’ knowledge, this is the first time MC-PCR was performed on raccoon meat.
Material and methods
Meat samples
Raccoons were gathered for sampling as previously detailed by Engel et al. (2022). Based on serological investigations performed previously by Engel et al. (2022), according to enzyme-linked immunosorbent assay (ELISA) results, animals were stratified into three groups. One group comprised seronegative animals with a sample to positive control (S/P) ratio < 8%. The other groups comprised either low positive animals with an S/P ratio between 30 and 60% or high positive ones with an S/P ratio > 120%. From each group, 50 raccoons were randomly chosen and sampled for molecular diagnostics. For sampling, each one previously frozen forelimb and hindlimb of every animal were defrosted for 2 days in a refrigeration chamber at 1 °C.
Magnetic-capture DNA extraction and real-time PCR
Samples were prepared and DNA was extracted by magnetic capture as described by Opsteegh et al. (2010) using minor modifications (Koethe et al. 2015) as well as some additional slight deviations. Approximately 100 g (67.1–116.2 g) meat sample (free of fat and connective tissue), which was composed of approximately 75% of the hindlimb and 25% of the forelimb due to meat availability, was used for further examination.
T. gondii tachyzoites (ME 49) were kindly provided from Institute of Parasitology, Faculty of Veterinary Medicine, Leipzig University, in Dulbecco Modified Eagle Medium (including 10% foetal bovine serum, 1% penicillin/streptomycin). To generate quantification standards and control samples for each magnetic-capture (MC) DNA extraction, meat from nine seronegative raccoons (S/P ratio < 4%) was demonstrated T. gondii DNA-free by MC-PCR, pooled, and split into 10-g aliquots. Tachyzoites were serially diluted in phosphate buffered saline (PBS) and added at a final concentration of 106 to 102 per 10 g of negative raccoon meat aliquot for quantification standards. For these standards, DNA was extracted by magnetic capture and thereupon included in every PCR run to calculate the amount of T. gondii in the examined sample. Additionally, for every MC-DNA extraction approach a 10-g meat aliquot was spiked with 103 tachyzoites to serve as positive extraction control. Another 10-g meat aliquot without tachyzoites was included as negative extraction control. By comparing the resulting sample cycle threshold value (Ct value) with the standards the quantity of T. gondii genome equivalents of each sample was calculated (StepOnePlus software, Life Technologies, Germany) and adjusted to determine the number of genome equivalents per 100 g of meat, expressed as log10 values. Qualitatively, MC-PCR results with Ct values < 35 were considered positive, and samples with Ct values > 40 were regarded as negative. From samples with Ct values between 35 and 40, the respective amplification curves were visually inspected and considered negative when no typical amplification course was observed.
Statistics
The statistical analyses were performed by Prism9 Software (GraphPad Software, LLC, USA). Low and high positive serostatus groups were compared by chi-square test regarding qualitative PCR results. To analyse for differences between these two groups in respect to genome equivalents per 100 g meat, quantitative PCR results were compared by t-test after confirmation of Gaussian data distribution. The Spearman correlation coefficient (rS) was determined to describe the relationship between S/P ratio and DNA equivalents present in 100 g meat. In general, p < 0.05 was regarded statistically significant.
Results
In 56 of 150 examined raccoon meat samples T. gondii DNA was detected by MC-PCR. All of the 50 serologically negative animals were also found to be negative for T. gondii DNA. Out of the serologically low positives (30–60% S/P ratio), 18 samples (36%) were MC-PCR positive, whilst 38 out of the 50 analysed samples from the high positive serostatus group (76%) were found to contain T. gondii DNA. Statistically, significantly more positive samples were detected in the high positive serostatus group (chi-square test, p < 0.0001). Detailed results are shown in Table 1.
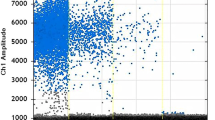
In samples with high S/P ratios statistically significant higher DNA equivalent values were detected compared to MC-PCR positive samples with lower S/P ratio (4.688 vs. 3.610 log10; p < 0.0001). In general, the amount of T. gondii DNA equivalents amongst the MC-PCR positive samples ranged from 2.622 log10 (S/P ratio of this sample: 30.02%) to 6.352 log10 (S/P ratio: 177%) per 100 g meat. Details on quantitative results are shown in Fig. 1.
Based on the quantitative MC-PCR results of the herein analysed samples, there was a positive correlation between S/P ratio and DNA equivalents (rS = 0.5439, p < 0.0001), which is illustrated in Fig. 2.
Discussion
Generally, in many studies T. gondii DNA was detected in samples from seropositive animals, including wildlife (Dubey 2022). Particularly for Germany, foxes (Vulpes vulpes) (Herrmann et al. 2012),wild boars (Sus scrofa), roe deer (Capreolus capreolus), and red deer (Cervus elaphus) were shown to contain T. gondii DNA (Stollberg et al. 2021). For raccoons, Kornacka et al. (2018) recently detected T. gondii DNA in typical predilection sites of seropositive animals by conventional PCR, especially in the brain but also in lungs and hearts. Based on only a few samples, they reported detection of T. gondii DNA in 50% (3/6) of serologically positive animals originating from Poland and Germany. Using the more sensitive MC-PCR method, we detected T. gondii DNA in meat samples of 56% (56/100) of serological positive raccoons. Further investigations in German wildlife made comparable findings, where approximately 52% (24/46) of wild boar and 41% (7/17) of roe deer samples were serological positive as well as DNA positive by PCR (Stollberg et al. 2021).
A higher agreement was detected in Canadian foxes with approximately 69% (11/16) of serological positives which were positive by MC-PCR as well (Nicholas et al. 2018). A much lower proportion of DNA positives in serological positive red foxes from Italy (approximately 8%, 8/102) was reported by Verin et al. (2013). Similar low agreements were found for German foxes by Herrmann et al. (2012), who observed DNA in 48 out of 301 (16%) serological positive animals by conventional PCR. Analysing German red deer, Stollberg et al. (2021) also found a comparable low proportion of PCR positive results on seropositive animals (25%). However, only single animals of red deer have been tested (n = 4) in that study. A high detection rate of DNA in serologically positive samples, e.g. as detected by Nicholas et al. (2018) with 69%, might be caused by different factors. They also used the sensitive MC-PCR method for DNA detection but examined tissues like brain and heart that are known to be predilection sites in many species (Juránková et al. 2014a, 2015; Koethe et al. 2015; Santoro et al. 2019), which might account for the higher detection rate compared to our results in skeletal muscle samples. MC-PCR is still not commonly used in animals or meat products, as it is a very laborious and expensive investigation. However, this type of DNA extraction was repeatedly found to be considerably more sensitive than conventional ones (Juránková et al. 2014b; Opsteegh et al. 2010). Using conventional PCR can, therefore, be a reason for lower DNA detection rates in seropositive animals.
To detect T. gondii DNA in meat products, especially for products that are usually uncooked, it is possible to apply MC-PCR as well. For example, it was successfully deployed for the examination of serrano ham, which was found to contain T. gondii DNA with a prevalence of approximately 9% (Gomez-Samblas et al. 2015). However, MC-PCR is performed mostly on predilection sites of T. gondii, which are expected to have a higher prevalence than examining skeletal muscle. Whilst this is reasonable for general parasitic investigations the aim of this study was to contribute knowledge on prevalence and quantity of T. gondii in raccoon meat parts that are relevant for potential consumption. Different sample material may also account for differences in DNA detection rates.
Additionally, differences on the level of agreement between serostatus and DNA prevalence may be due to the corresponding antibody level and the applied method of serological examination. In this study, animals were stratified according to serological status into serological low positive and high positive based on previous ELISA results (Engel et al. 2022). Different serological methods may include distinct discrimination levels, which could lead to a different categorization of serological results and, thus, to a different level of agreement. To date, the dynamics of antibody response following contact with the parasite in raccoons are not well known. Thus, the extent to which a current serostatus information is reliable for the infection status of a raccoon is uncertain.
All serologically negative raccoons examined in this study also proved negative in MC-PCR. For raccoons, Kornacka et al. (2018) identified 44% (15/34) serologically negative animals to contain T. gondii DNA in animal predilection sites, which is contrary to our observations, but is similar to previous investigations for other animals. Nicholas et al. (2018) found 30% (7/23) of serological negative foxes in Canada to be positive by MC-PCR for T. gondii DNA. This might be because they examined the predilection sites of animals, where tissue cysts are usually most common. Furthermore, we only investigated animals with a very low S/P ratio (< 8%) for the seronegative group, whilst all animals with an S/P ratio < 25% are regarded negative according to the manufacturers’ instructions. Kornacka et al. (2018) used the same test and it must be assumed that seronegative animals from their study have a broader S/P range compared to the seronegative animals in our study. Similar findings were made previously for other animals, using serological examinations and bioassay. Seronegative pigs rarely also harboured infectious T. gondii stages (Dubey et al. 1995) which was later discussed to be because of a very recent infection or of a decrease of antibodies to an undetectable level (Dubey 2022).
In this study, we detected a positive correlation between antibody level and presence of T. gondii DNA. For 36% of the serologically low positive animals and for 76% of the serologically high positive animals, T. gondii DNA could be detected. Similar observations were made for other species as well. In chickens, a correlation was observed between serological status and pathogen isolation. The frequency of T. gondii isolation increased sharply with rising MAT titres; whilst only 61% were isolated at low titres, it was already up to 75% at high titres using a bioassay (Dubey et al. 2016). This leads to the conclusion that higher seroprevalence titres are related to higher parasite loads (Dubey 2022).
However, results strongly depend on the examined tissue, DNA extraction method, PCR method (Dubey 2022), and the amount examined, as it is known that T. gondii is not homogeneously distributed in organisms (Opsteegh et al. 2010). For detection of infectious parasites, a bioassay, e.g. together with a cell culture, is necessary which is very time-consuming, associated with high costs, and requires the use of laboratory animals (Algaba et al. 2017; Dubey 2022). Using PCR, only DNA detections are obtained. The material costs of MC-PCR are higher than for conventional DNA extraction, which is usually performed with a special kit but they are not as high as for a bioassay (Opsteegh et al. 2010). Thus, MC-PCR can be used as an alternative to bioassay in respect to detection and genotyping of T. gondii, and to quantify the organism in meat samples of various source (Opsteegh et al. 2010).
For wild animals in Germany, the examination of tissue by MC-PCR showed the best agreement for DNA detection in relation to serological examinations (Stollberg et al. 2021). For sheep and lambs, there was a statistically significant increase in the probability of isolating T. gondii in skeletal muscle with increasing sample size per animal (Rani et al. 2020). Since in conventional PCR only a few milligrams or up to a few grams of meat can be examined, an MC-PCR has a significantly higher probability for detecting parasite DNA when using up to 100 g meat.
Finally, we observed skeletal muscle tissue as meat for potential human consumption. Since for different animal species brain and heart were found to be the main predilection sites for T. gondii tissue cysts (Juránková et al. 2014a, 2015; Koethe et al. 2015; Santoro et al. 2019) we may underestimate the general prevalence of T. gondii DNA in raccoons. However, the focus of this study rather was the detection of parasite burden in consumption relevant parts of raccoons to provide knowledge for further public health evaluations on this kind of meat. We found that meat from seropositive raccoons may contain up to about 6 log10 of T. gondii equivalents. Based on the modelling of Guo et al. (2016) this number would be sufficient for a high probability of infection in humans.
Conclusion
Previous seroepidemiological research proved a high presence of T. gondii in German raccoons. In addition, we showed a high detection rate of T. gondii DNA in meat from seropositive raccoons. Seropositive animals could harbour considerable numbers of T. gondii, where antibody titre is positively correlated with DNA amount. If the parasites in meat are also likely to be infective, appropriate care must be taken to avoid infection and the meat has to be sufficiently heated before consumption. To better assess the public health risk posed by raccoon meat, further investigations, such as bioassay for evaluation of parasite infectivity, need to be performed.
Data availability
The data related to the manuscript will be available upon request to the corresponding author.
References
Algaba IG, Geerts M, Jennes M, Coucke W, Opsteegh M, Cox E, Dorny P, Dierick K, de Craeye S (2017) A more sensitive, efficient and ISO 17025 validated magnetic capture real time PCR method for the detection of archetypal Toxoplasma gondii strains in meat. Int J Parasitol 47:875–884. https://doi.org/10.1016/j.ijpara.2017.05.005
Beltrán-Beck B, García FJ, Gortázar C (2012) Raccoons in Europe: disease hazards due to the establishment of an invasive species. Eur J Wildl Res 58:5–15. https://doi.org/10.1007/s10344-011-0600-4
Berger-Schoch AE, Bernet D, Doherr MG, Gottstein B, Frey CF (2011) Toxoplasma gondii in Switzerland: a serosurvey based on meat juice analysis of slaughtered pigs, wild boar, sheep and cattle. Zoonoses Publ Health 58:472–478. https://doi.org/10.1111/j.1863-2378.2011.01395.x
Bier NS, Stollberg K, Mayer-Scholl A, Johne A, Nöckler K, Richter M (2020) Seroprevalence of Toxoplasma gondii in wild boar and deer in Brandenburg, Germany. Zoonoses Publ Health 67:601–606. https://doi.org/10.1111/zph.12702
Burger J (2000) Gender differences in meal patterns: role of self-caught fish and wild game in meat and fish diets. Environ Res 83:140–149. https://doi.org/10.1006/enrs.2000.4060
Dubey JP, Thulliez P, Weigel RM, Andrews CD, Lind P, Powell EC (1995) Sensitivity and specificity of various serologic tests for detection of Toxoplasma gondii infection in naturally infected sows. Am J Vet Res 56:1030–1036
Dubey JP, Sundar N, Hill D, Velmurugan GV, Bandini LA, Kwok OCH, Majumdar D, Su C (2008) High prevalence and abundant atypical genotypes of Toxoplasma gondii isolated from lambs destined for human consumption in the USA. Int J Parasitol 38:999–1006. https://doi.org/10.1016/j.ijpara.2007.11.012
Dubey JP, Laurin E, Kwowk OCH (2016) Validation of the modified agglutination test for the detection of Toxoplasma gondii in free-range chickens by using cat and mouse bioassay. Parasitology 143:314–319. https://doi.org/10.1017/S0031182015001316
Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH (2021) Recent epidemiologic and clinical Toxoplasma gondii infections in wild canids and other carnivores: 2009–2020. Vet Parasitol 290:109337. https://doi.org/10.1016/j.vetpar.2020.109337
Dubey JP (2022) Toxoplasmosis of animals and humans. CRC Press, Boca Raton, London, New York
Engel L, Hamedy A, Kornacka-Stackonis A, Langner T, Birka S, Koethe M (2022) Toxoplasma gondii in raccoons (Procyon lotor) in Germany: a serosurvey based on meat juice. Parasitol Res 121:3417–3425. https://doi.org/10.1007/s00436-022-07646-w
Gaines KF, Lord CG, Boring CS, Brisbin IL, Gochfeld M, Burger J (2000) Raccoons as potential vectors of radionuclide contamination to human food chains from a nuclear industrial site. J Wildl Manag 64:199. https://doi.org/10.2307/3802991
Gamble HR, Dubey JP, Lambillotte DN (2005) Comparison of a commercial ELISA with the modified agglutination test for detection of Toxoplasma infection in the domestic pig. Vet Parasitol 128:177–181. https://doi.org/10.1016/j.vetpar.2004.11.019
German Hunting Association (2019) Deutschland liebt Wildbret vom Wildschwein. https://www.jagdverband.de/deutschland-liebt-wildbret-vom-wildschwein. Accessed 20 January 2022
German Hunting Association (2020) Ein bärig guter Braten? https://www.jagdverband.de/ein-baerig-guter-braten. Accessed 20 January 2022
German Hunting Association (2021) DJV-Handbuch Jagd 2021. DJV-Service GmbH, Bonn
Goguen AD, Riley SJ (2020) Consumption of wild-harvested meat in society. Wildl Soc Bull 44:553–563. https://doi.org/10.1002/wsb.1108
Gomez-Samblas M, Vílchez S, Racero JC, Fuentes MV, Osuna A (2015) Quantification and viability assays of Toxoplasma gondii in commercial “Serrano” ham samples using magnetic capture real-time qPCR and bioassay techniques. Food Microbiol 46:107–113. https://doi.org/10.1016/j.fm.2014.07.003
Guo M, Mishra A, Buchanan RL, Dubey JP, Hill DE, Gamble HR, Jones JL, Du X, Pradhan AK (2016) Development of dose-response models to predict the relationship for human Toxoplasma gondii infection associated with meat consumption. Risk Anal 36:926–938. https://doi.org/10.1111/risa.12500
Halos L, Thébault A, Aubert D, Thomas M, Perret C, Geers R, Alliot A, Escotte-Binet S, Ajzenberg D, Dardé M-L, Durand B, Boireau P, Villena I (2010) An innovative survey underlining the significant level of contamination by Toxoplasma gondii of ovine meat consumed in France. Int J Parasitol 40:193–200. https://doi.org/10.1016/j.ijpara.2009.06.009
Heddergott M, Frantz AC, Stubbe M, Stubbe A, Ansorge H, Osten-Sacken N (2017) Seroprevalence and risk factors of Toxoplasma gondii infection in invasive raccoons (Procyon lotor) in Central Europe. Parasitol Res 116:2335–2340. https://doi.org/10.1007/s00436-017-5518-7
Heddergott M, Müller F (2020) Hohe Prävalenz von Antikörpern gegen Toxoplasma gondii im Blutserum von Waschbären (Procyon lotor) aus der nordwestlichen hessischen Rhön, Deutschland. Beiträge Zur Jagd- Und Wildforschung 45:125–132
Herrmann DC, Maksimov P, Maksimov A, Sutor A, Schwarz S, Jaschke W, Schliephake A, Denzin N, Conraths FJ, Schares G (2012) Toxoplasma gondii in foxes and rodents from the German Federal States of Brandenburg and Saxony-Anhalt: seroprevalence and genotypes. Vet Parasitol 185:78–85. https://doi.org/10.1016/j.vetpar.2011.10.030
Hosein S, Limon G, Dadios N, Guitian J, Blake DP (2016) Toxoplasma gondii detection in cattle: a slaughterhouse survey. Vet Parasitol 228:126–129. https://doi.org/10.1016/j.vetpar.2016.09.001
Jones JL, Dubey JP (2012) Foodborne toxoplasmosis. Clin Infect Dis 55:845–851. https://doi.org/10.1093/cid/cis508
Jones JL, Parise ME, Fiore AE (2014) Neglected parasitic infections in the United States: toxoplasmosis. Am J Trop Med Hyg 90:794–799. https://doi.org/10.4269/ajtmh.13-0722
Juránková J, Opsteegh M, Neumayerová H, Kovařčík K, Frencová A, Baláž V, Volf J, Koudela B (2013) Quantification of Toxoplasma gondii in tissue samples of experimentally infected goats by magnetic capture and real-time PCR. Vet Parasitol 193:95–99. https://doi.org/10.1016/j.vetpar.2012.11.016
Juránková J, Basso W, Neumayerová H, Baláž V, Jánová E, Sidler X, Deplazes P, Koudela B (2014) Brain is the predilection site of Toxoplasma gondii in experimentally inoculated pigs as revealed by magnetic capture and real-time PCR. Food Microbiol 38:167–170. https://doi.org/10.1016/j.fm.2013.08.011
Juránková J, Hůrková-Hofmannová L, Volf J, Baláž V, Piálek J (2014) Efficacy of magnetic capture in comparison with conventional DNA isolation in a survey of Toxoplasma gondii in wild house mice. Eur J Protistol 50:11–15. https://doi.org/10.1016/j.ejop.2013.08.002
Juránková J, Basso W, Neumayerová H, Frencová A, Baláž V, Deplazes P, Koudela B (2015) Predilection sites for Toxoplasma gondii in sheep tissues revealed by magnetic capture and real-time PCR detection. Food Microbiol 52:150–153. https://doi.org/10.1016/j.fm.2015.07.005
Kauhala K (1996) Introduced carnivores in Europe with special reference to central and northern Europe. Wildl Biol 2:197–204. https://doi.org/10.2981/wlb.1996.019
Koethe M, Straubinger RK, Pott S, Bangoura B, Geuthner A-C, Daugschies A, Ludewig M (2015) Quantitative detection of Toxoplasma gondii in tissues of experimentally infected turkeys and in retail turkey products by magnetic-capture PCR. Food Microbiol 52:11–17. https://doi.org/10.1016/j.fm.2015.06.005
Kornacka A, Cybulska A, Popiołek M, Kuśmierek N, Moskwa B (2018) Survey of Toxoplasma gondii and Neospora caninum in raccoons (Procyon lotor) from the Czech Republic, Germany and Poland. Vet Parasitol 262:47–50. https://doi.org/10.1016/j.vetpar.2018.09.006
Lundén A, Lind P, Engvall EO, Gustavsson K, Uggla A, Vågsholm I (2002) Serological survey of Toxoplasma gondii infection in pigs slaughtered in Sweden. Scand J Infect Dis 34:362–365. https://doi.org/10.1080/00365540110080205
Lutz W (1995) Occurrence and morphometrics of the raccoon Procyon lotor L. in Germany. Ann Zool Fennici 32:15–20
Nicholas B, Ravel A, Leighton P, Stephen C, Iqbal A, Ndao M, Konecsni K, Fernando C, Jenkins E (2018) Foxes (Vulpes vulpes) as sentinels for parasitic zoonoses, Toxoplasma gondii and Trichinella nativa, in the northeastern Canadian Arctic. Int J Parasitol Parasites Wildl 7:391–397. https://doi.org/10.1016/j.ijppaw.2018.10.003
Opsteegh M, Langelaar M, Sprong H, den Hartog L, de Craeye S, Bokken G, Ajzenberg D, Kijlstra A, van der Giessen J (2010) Direct detection and genotyping of Toxoplasma gondii in meat samples using magnetic capture and PCR. Int J Food Microbiol 139:193–201. https://doi.org/10.1016/j.ijfoodmicro.2010.02.027
Opsteegh M, Teunis P, Züchner L, Koets A, Langelaar M, van der Giessen J (2011) Low predictive value of seroprevalence of Toxoplasma gondii in cattle for detection of parasite DNA. Int J Parasitol 41:343–354. https://doi.org/10.1016/j.ijpara.2010.10.006
Račka K, Bártová E, Budíková M, Vodrážka P (2015) Survey of Toxoplasma gondii antibodies in meat juice of wild boar (Sus scrofa) in several districts of the Czech Republic. Ann Agric Environ Med 22:231–235. https://doi.org/10.5604/12321966.1152071
Rani S, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Dubey JP, Pradhan AK (2020) Distribution of Toxoplasma gondii tissue cysts in shoulder muscles of naturally infected goats and lambs. J Food Prot 83:1396–1401. https://doi.org/10.4315/JFP-20-024
Salgado I (2018) Is the raccoon (Procyon lotor) out of control in Europe? Biodivers Conserv 27:2243–2256. https://doi.org/10.1007/s10531-018-1535-9
Santoro M, Viscardi M, Sgroi G, D’Alessio N, Veneziano V, Pellicano R, Brunetti R, Fusco G (2019) Real-time PCR detection of Toxoplasma gondii in tissue samples of wild boars (Sus scrofa) from southern Italy reveals high prevalence and parasite load. Parasit Vectors 12:335. https://doi.org/10.1186/s13071-019-3586-5
Stollberg KC, Schares G, Mayer-Scholl A, Hrushetska I, Diescher S, Johne A, Richter MH, Bier NS (2021) Comparison of direct and indirect Toxoplasma gondii detection and genotyping in game: relationship and challenges. Microorganisms 9:1663. https://doi.org/10.3390/microorganisms9081663
Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30:1217–1258. https://doi.org/10.1016/S0020-7519(00)00124-7
Verin R, Mugnaini L, Nardoni S, Papini RA, Ariti G, Poli A, Mancianti F (2013) Serologic, molecular, and pathologic survey of Toxoplasma gondii infection in free-ranging red foxes (Vulpes vulpes) in central Italy. J Wildl Dis 49:545–551. https://doi.org/10.7589/2011-07-204
Acknowledgements
We are grateful to Zaida Melina Renteria Solis from the Institute of Parasitology, Faculty of Veterinary Medicine, Leipzig University for providing the T. gondii tachyzoites.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors made substantial contribution to the conception and design of the study. L. E. collected the data. M. K. and L. E. analysed and interpreted the data. L. E. has written the first draft of the manuscript and all authors revised it critically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Nawal Hijjawi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Engel, L., Hamedy, A. & Koethe, M. Direct detection and quantification of Toxoplasma gondii in meat samples from feral raccoons (Procyon lotor) in Germany by magnetic-capture real-time PCR. Parasitol Res 122, 307–313 (2023). https://doi.org/10.1007/s00436-022-07730-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07730-1