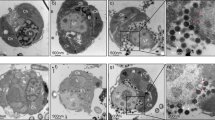
Abstract
As both groups of organisms, free-living amoebae (FLA) and viruses, can be found in aquatic environments side by side, it appears obvious that there are multiple interactions with respect to host—endocytobiont relationships. Several relationships between viruses and protozoan hosts are described and it was the discovery of the so called “giant viruses,” associated with amoebae, which gave another dimension to these interactions. Mimiviruses, Pandoraviruses and Pithoviruses are examples for interesting viral endocytobionts within FLA. In the Mimivirus viral factories, viral DNA undergoes replication and transcription, and the DNA is prepared to be packed in procapsids. Theses Mimivirus factories can be considered as efficient “production lines” where, at any given moment, all stages of viral generation including membrane biogenesis, capsid assembly and genome encapsidation, are occurring concomitantly. There are some hints that similar replication factories are involved as well during the Pandoravirus development. Some scientists favour the assumption that the giant viruses have received many of their genes from their hosts or from sympatric occurring endocytobionts via lateral gene transfer. This hypothesis would mean that this type of transfer has been an important process in the evolution of genomes in the context of the intracellular parasitic or endocytobiotic lifestyle. In turn, that would migitate against hypothesizing development of a new branch in the tree of life. Based on the described scenarios to explain the presence of genes related to translation, it is also possible that earlier ancestors of today’s DNA viruses were involved in the origin of eukaryotes. That possibly could in turn support the idea that cellular organisms could have evolved from viruses with growing autarkic properties. In future we expect the discovery of further (giant) viruses within free-living amoebae and other protozoa through genomic, transcriptomic and proteomic analyses.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Abrahao J, Dornas F, Silva L, Almeida G, Boratto P, Colson P, La Scola B, Kroon E (2014) Acanthamoeba polyphaga Mimivirus and other giant viruses: an open field to outstanding discoveries. Virology Journal 11:120
Antwerpen M, Georgi E, Zoeller L, Woelfel R, Stoecker K, Scheid P (2015) Whole-genome sequencing of a Pandoravirus isolated from keratitis-inducing Acanthamoeba. Genome Announc 3(2):e00136–15. doi:10.1128/genomeA.00136-15
Bandea C (1983) A new theory on the origin and the nature of viruses. J Theor Biol 105:591–602
Bandea C (2009) The origin and evolution of viruses as molecular organisms. Nature proceedings: hdl:10101/npre.2009.3886.1
Barker J, Lambert P, Brown M (1992) Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun 61:3503–3510
Baron D, Danglot C, Vilagines R (1980) Role of a free-living amoeba from water, Acanthamoeba castellanii, in the transport of naked or enveloped animal viruses. CR Seances Acad Sci D 291; 629–632
Battistini R, Marcucci E, Verania M, Di Guiseppe G, Dini F, Carduccia A (2013) Ciliate-Adenovirus interactions in experimental co-cultures of Eupletes octocarinatus and in wastewater environment. Eur J Protistol 49:381–388
Boyer M, Yutin N, Pagnier I (2009) Giant Marseillevirus highlights the role of amoebae as a melting pot in emergence of chimeric microorganisms. Proc Natl Acad Sci 106:21848–22853
Chelikani V, Ranjan T, Kondabagil K (2014a) Revisiting the genome packaging in viruses with lessons from the “Giants”. Virology 466-467:15-26
Chelikani V, Ranjan T, Zade A, Shukla A, Kondabagil K (2014b) Genome segregation and packaging machinery in Acanthamoeba polyphaga Mimivirus is reminiscent of bacterial apparatus. J Virol 88: 6069-6075
Claverie J (2006) Viruses take center stage in cellular evolution. Genome Biol 7:110
Claverie J, Abergel C (2011) Family Mimiviridae. Virus Taxonomy, ninth report of the International Committee on Taxonomy of Viruses (ICTV), Elsevier, Academic Press, ISBN : 978-0-12-384684-6; 223–228
Colson P, Raoult D (2010) Gene repertoire of amoeba-associated giant viruses. Intervirology 53(5):330–343
Colson P, De Lamballerie X, Fournous G, Raoult D (2012) Reclassification of giant viruses composing a fourth domain in the new order Megavirales. Intervirology 55:321–332
Colson P, La Scola B, Raoult D (2013) Giant viruses of amoebae as potential human pathogens. Intervirology 56:376–385
Danes L, Cerva L (1981) Survival of Polioviruse and Echoviruses in Acanthamoeba castellanii cultivated in vitro. J Hyg Epidemiol Microbiol Immunol 25(2):169–174
Dare R, Chittaganpitch M, Erdman D (2008) Screening pneumonia patients for Mimivirus. Emerg Infect Dis 14:465–467
De Silva F, Moss B (2005) Origin-independent plasmid replication occurs in vaccinia virus cytoplasmic factories and requires all five known poxvirus replication factors. Virol J doi: 10.186/1743-422X-2-23
Desnues C, La Scola B, Yutin N, Fournous G, Robert C, Azza S, Jardot P, Monteil S, Campocasso A, Koonin E, Raoult D (2012) Provirophages and transportvirons as the diverse mobilome of giant viruses. Proc Natl Acad Sci USA 109:18078–18083
Diamond L, Mattern C (1976) Protozoal viruses. Adv Virus Res 20:87–112
Dunnebacke T, Schuster F (1971) Infectious agent from a free-living soil amoeba, Naegleria gruberi. Science 174:516–518
Dunnebacke T, Schuster F (1974) An infectious agent associated with amebas oft he genus Naegleria. J Protozool 21:327–329
Fischer M, Allen M, Wilson W, Suttle C (2010) Giant virus with a remarkable complement of genes infects marine zooplankton. Proc Natl Acad Sci USA 107:19508–19513
Gajdatsy A, Kosmin A, Barrett G (2008) Coexistent adenoviral keratoconjunctivitis and Acanthamoeba keratitis; Clin & Exp Ophtal; doi: 10.1046/j.1442-9071.2000.00352.x
Ghigo E, Kartenbeck J, Lien P, Pelkmans L, Capo C, Mege J-L, Raoult D (2008) Amebal pathogen Mimivirus infects macrophages through phagocytosis. PLoS Pathogens 4, e1000087
Greub G, Raoult D (2004) Microorganisms resistant to free-living amoebae. Clin Microbiol Rev 17:413–433
Hoffmann R, Michel R, Müller K-D, Schmid E (1998) Archaea-like endocytobiotic organisms isolated from Acanthamoeba sp. (Gr II). Endo Cell Res 12:185–188
Hsueh T, Gibson K (2015) Interactions between Human Norovirus Surrogates and Acanthamoeba spp.. Appl Environ Microbiol. 81(12):4005-13. doi: 10.1128/AEM.00649-15. Epub 2015 Apr 3.
Khan N (2009) Acanthamoeba, biology and pathogenesis. Caister Academic Press, Norfolk
Klose T, Kuznetsov Y, Xiao C, Sun S, McPherson A, Rossmann M (2010) The three-dimensional structure of Mimivirus. 53; 268–273; DOI: 10.1159/000312911
Koonin E (2005) Virology; Gulliver among the Lilliputians. Curr Biol 15:167–169
La Scola B, Audic S, Robert C, Jungjang L, De Lamballerie X, Drancourt M, Birtles R, Claverie J, Raoult D (2003) A giant virus in amoebae. Science 299:5615
La Scola B, Marrie T, Auffray J, Raoult D (2005) Mimivirus in pneumonia patients. Emerg Infect Dis 11:449–452
La Scola B, Desnues C, Pagnier I, Robert C, Barassi L, Fournous G, Merchat M, Suzan-Monti M, Forterre P, Koonin E, Raoult D (2008) The virophage as a unique parasite of the giant Mimivirus. Nature 445:100–105
La Scola B, Campocasso A, N’dong R, Fournous G, Barrassi L, Flaudrops C, Raoult D (2010) Tentative characterization of new environmental giant viruses by MALDI-TOF mass spectrometry. Intervirology 53:344–353
Lamrabet O, Merhej V, Pontarotti P, Raoult D, Drancourt M (2012) The genealogic tree of Mycobacteria reveals a long standing sympatric life into free-living protozoa. PLoS ONE 7, e34754
Legendre M, Bartoli J, Shmakova L, Jeudy S, Labadie K, Adrait A, Lescot M, Poirot O, Bertaux L, Bruley C, Coute Y, Rivkina E, Abergel C, Claverie J (2014) Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a Pandoravirus morphology. PNAS 111:4274–4279
Lorenzo-Morales J, Coronado-Alvarez N, Martinez-Carretero E, Maciver S, Valladares B (2007) Detection of four Adenovirus serotypes within water-isolated strains of Acanthamoeba in the Canary Islands, Spain. Am J Trop Med Hyg 77(4):753–756
Margulis L, Schwartz K (1989) Die fünf Reiche der Organismen. Ein Leitfaden. Spektrum-der-Wissenschaft-Verlagsgesellschaft mbH & Co, Heidelberg
Mattana A, Serra C, Mariotti E, Delogu G, Fiori P, Cappucinelli P (2006) Acanthamoeba castellanii promotion of in vitro survival and transmission of Coxsackie B3 viruses. Eukaryotic Cel 5(4):665–671
Michel R, Müller K-D, Schmid E, Zöller L, Hoffmann R (2003) Endocytobiont KC 5/2 induces transformation into sol-like cytoplasm of its host Acanthamoeba sp. as substrate for his own development. Parasitol Res 90:52–56
Miller J, Swartzwelder J (1960) Virus-like particles in an Entamoeba histolytica trophozoite. Parasite 46:523–524
Moliner C, Raoult D, Fournier P (2009) Evidence that intra-amoebal Legionella drancourtii acquired a sterol reductase gene from eukaryotes. BMC Res Notes 2:51
Molmeret M, Horn M, Wagner M, Santic M, Kwaik Y (2005) Amoebae as training grounds for intracellular bacterial pathogens. Appl Environ Microbiol 71:20–28
Moreira D, Brochier-Armanet C (2008) Giant viruses, giant chimeras: the multiple evolutionary histories of Mimivirus genes. BMC Evol Biol 8:12. doi:10.1186/1471-2148-8-12
Mutsafi Y, Zauberman N, Sabanay I, Minsky A (2010) Vaccinia-like cytoplasmic replication oft he giant Mimivirus. Proc Natl Acad Sci USA 107:5978–5982
Mutsafi Y, Shimoni E, Shimion A, Minsky A (2013) Membrane assembly during the infection cycle of the giant Mimivirus. PLOS Pathogens 9:e1003367
Ngounga T, Pagnier I, Reteno D, Raoult D, La Scola B, Colson P (2013) Real-time PCR targeting giant viruses of amoebae and their virophages. Intervirology 56:413–423
Oliveira S, Costa J (1995) Replication of transfected plasmid DNA by cells infected with African swine fever virus. Virology 207:392–399
Philippe N, Legendre M, Doutre G, Coute Y, Poirot O, Lescot M, Arslan D, Seltzer V, Bertraux L, Bruley C, Garin J, Claverie J, Abergel C (2013) Pandoraviruses: amoeba viruses with genomes up to 2.5 Mb reaching that of parasitic eukaryotes. Science 341:281–286
Popgeorgiev N, Boyer M, Fancello L, Monteil S, Robert C, Rivet R, Nappez C, Azza S, Chiaroni J, Raoult D, Desnues C (2013) Marseillevirus-like virus recovered from blood donated by asymptomatic humans. J Infect Dis 208:1042–1050. doi:10.1093/infdis/jit292
Raoult D, Boyer M (2010) Amoebae as genitors and reservoirs of giant viruses. Intervirology 53:321–329
Raoult D, Forterre P (2008) Redefining viruses: lessons from Mimivirus. Nat Rev Microbiol 6:315–319
Raoult D, Audic S, Robert C, Abergel C, Renesto P, Ogata H, La Scola B, Suzan M, Claverie J (2004) The 1.2-megabase genome sequence of Mimivirus. Science 306:1344–1350
Raoult D, Renesto P, Brouqui P (2006) Laboratory infection of a technician by Mimivirus. Ann Intern Med 144(9):702–703
Raoult D, La Scola B, Birtles R (2007) The discovery and characterization of Mimivirus, the largest known virus and putative pneumonia agent. Emerg Inf 45:95–101
Saadi H, Pagnier I, Colson P, Cherif J, Beji M, Boughalmi M, Azza S, Armstrong N, Robert C, Fournous G, La Scola B, Raoult D (2013) First isolation of Mimivirus in a patient with pneumonia. Clin Infect Dis; 57; doi: 10.1093/cid/cit354
Scheid P (2008) Freilebende Amöben als Krankheitserreger und Vektoren. Wehr Med Mo 52:171–175
Scheid P (2014) Relevance of free-living amoebae as hosts for phylogenetically diverse microorganisms. Parasitol Res 113:2407–2414. doi:10.1007/s00436-014-3932-7
Scheid P, Schwarzenberger R (2012) Acanthamoeba spp. as vehicle and reservoir of adenoviruses; Parasitol Res; doi 10.1007/s00436-012-2828-7
Scheid P, Pressmar S, Richard G, Zöller R, Michel R (2008) An extraordinary endocytobiont in Acanthamoeba sp. isolated from a patient with keratitis. Parasitol Res 102:945–950
Scheid P, Hauroeder B, Michel R (2010) Investigations of an extraordinary endocytobiont in Acanthamoeba sp.: development and replication. Parasitol Res 106:1371–1377
Scheid P, Balczun C, Schaub G (2014) Some secrets are revealed: parasitic keratitis amoebae as vectors of the scarcely described Pandoraviruses to humans. Parasitol Res 113(10):3759–3764. doi:10.1007/s00436-014-4041-3
Schuster F (1969) Intranuclear virus-like bodies in the amoeboflagellate Naegleria gruberi. J Protozool 16:724–727
Selinka H, Botzenhart K, Feuerpfeil I, Puchert W, Schmoll O, Szewzyk R, Willmitzer H (2011) Nachweis von Viren im Rohwasser als Grundlage einer Risikoabschätzung. Bundesgesundheitsbl 54:496–504
Sharma V, Colson P, Giorgi R, Pontarotti P, Raoult D (2014) DNA-dependent RNA polymerase detects hidden giant viruses in published databanks. Genome Biol Evol 6:1–22. doi:10.1093/gbe/evu128
Sharma V, Colson P, Chabrol O, Scheid P, Pontarotti P, Raoult D (2015a) Welcome to pandoraviruses at the “Fourth TRUC” club. Front Microbiol 6:423. doi:10.3389/fmicb.2015.00423
Sharma V, Colson P, Chabrol O, Pontarotti P, Raoult D (2015b) Pithovirus sibericum, a new bona fide member of the “Fourth TRUC” club. Front Microbiol 6:722. doi:10.3389/fmicb.2015.00722
Suzan-Monti M, La Scola B, Barrassi L, Espinosa L, Raoult D (2007) Ultrastructural characterization of the giant volcano-like virus factory of Acanthamoeba polyphaga Mimivirus. PLoS ONE 2:e328. doi:10.1371/journal.pone.0000328
Thomas V, Greub G (2010) Amoeba/Amoebal symbiont genetic transfers: lessons from giant virus neighbours. Intervirology 53:254–267
Van Regenmortel M (2000) 7th report of the international committee on the taxonomy of viruses. Academic, San Diego, pp 3–16
Woese C, Kandler O, Wheelis M (1990) Towards a natural sytem of organisms: proposal for the domains Archaea, Bacteria and Eucarya. Proc Natl Acad Sci USA 87:4576–4579
Yoosuf N, Yutin N, Colson P, Shabalina S, Pagnier I, Robert C, Azza S, Klose T, Wong J, Rossmann M, La Scola B, Raoult D, Koonin E (2012) Related giant viruses in distinct locations and different habitats: Acanthamoeba polyphaga Moumouvirus represents a third lineage of the Mimiviridae that is close to the megavirus lineage. Genome Biol Evol 4:1324–1330
Yutin N, Koonin E (2013) Pandoraviruses are highly derived phycodnaviruses. Biol Direct 8:25. doi:10.1186/1745-6150-8-25
Zauberman N, Mutsafi Y, Havely D, Shimoni E, Klein E, Xiao C, Sund S, Minsky A (2008) Distinct DNA exit and packaging portals in the virus Acanthamoeba polyphaga Mimivirus. PLoS Biol 6:e114, 1104–1114
Acknowledgements
I want to thank B. Hauroeder and team (ZInstSanBw Koblenz) and Lars Möller (RKI) for the outstanding EM-photos. I thank Albrecht Kiderlen and Rolf Michel for their fruitful discussions, David Lam (Shaman Medical Consulting) for review and English-language editing of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheid, P. Viruses in close associations with free-living amoebae. Parasitol Res 114, 3959–3967 (2015). https://doi.org/10.1007/s00436-015-4731-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4731-5