Abstract
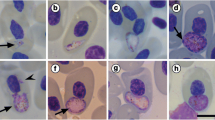
Plasmodium (Novyella) unalis sp. nov. was found in the Great Thrush, Turdus fuscater (Passeriformes, Turdidae) in Bogotá, Colombia, at 2,560 m above sea level where the active transmission occurs. This parasite is described based on the morphology of its blood stages and a fragment of the mitochondrial cytochrome b gene (lineage UN227). Illustrations of blood stages of new species are given, and the phylogenetic analysis identifies closely related species and lineages of avian malaria parasites. The new species is most similar to Plasmodium (Novyella) vaughani (lineage SYAT05), a cosmopolitan avian malaria parasite; these parasites are also closely related genetically, with a genetic difference of 3.2 % between them. P. unalis can be readily distinguished from the latter species morphologically, primarily due to the (1) presence of a single large, circular shaped pigment granule in the erythrocytic trophozoites and meronts; (2) presence of prominent vacuoles in trophozoites and growing meronts; and (3) presence of predominantly fan-like shaped erythrocytic meronts. Cytochrome b lineages with high similarity to the new species have been reported in Costa Rica, Brazil, Chile, and USA. It is probable that the new species of malaria parasite is widely distributed in the New World. This parasite has been reported only in the Great Thrush at the study site and might have a narrow range of avian hosts. Records of P. unalis are of particular theoretical interest due to its active transmission at highlands in Andes. Possible influence of urbanization on transmission of this malaria parasite in Bogotá is discussed.




Similar content being viewed by others
References
Angel L, Ramírez A, Dominguez E (2010) Heat island and temperature spatiotemporal changes in Bogotá City. Rev Acad Colomb Cienc Exact Fis Nat 34:173–183
Asociacion Bogotana de Ornitologia (ed) (2000) Aves de la Sabana de Bogota, guia de campo. ABO CAR, Bogota
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hannson B, Westerdahl H, Pinheiro RT (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc Lond B Biol Sci 267:1583–1589. doi:10.1098/rspb.2000.1181
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
Bensch S, Pérez-Tris J, Waldenström J, Hellgren O (2004) Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution 58:1617–1621. doi:10.1111/j.0014-3820.2004.tb01742.x
Chavatte JM, Uzbekov R, Paperna I, Richard-Lenoble D, Landau I (2010) Ultrastructure of erythrocytic stages of avian Plasmodium spp. of the sub-genus Novyella and its “globule”. Parasite 17:123–127. doi:10.1051/parasite/2010172123
Corradetti A, Garnham PCC, Laird M (1963) New classification of the avian malaria parasites. Parassitologia 5:1–4
Corradetti A, Scanga M (1965) Notes on Plasmodium (Giovannolaia) polare and its transmission with Culiseta longiareolata. Parassitologia 7:61–64
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. doi:10.1038/nmeth.2109
Department of Geosciences Universidad Nacional de Colombia (2011) Boletín del análisis de las variables obtenidas en la estación meteorológica de la Universidad Nacional. http://www.geociencias.unal.edu.co/?niv=not¬=545&dep=12. Accessed 30 Apr 2013
Dodge M, Guers SL, Sekercioğlu ÇH, Sehgal RNM (2013) North American transmission of hemosporidian parasites in the Swainson’s thrush (Catharus ustulatus), a migratory songbird. J Parasitol 99:548–553. doi:10.1645/GE-3134.1
Freed LA, Cann RL, Goff ML, Kuntz WA, Bodner GR (2005) Increase in avian malaria at upper elevation in Hawai’i. Condor 107:753–764. doi:10.1650/7820.1
Glaizot O, Fumagalli L, Iritano K, Lalubin F, Van Rooyen J, Christe P (2012) High prevalence and lineage diversity of avian malaria in wild populations of Great Tits (Parus major) and mosquitoes (Culex pipiens). PLoS ONE 7:e34964. doi:10.1371/journal.pone.0034964
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319:756–760. doi:10.1126/science.1150195
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hellgren O, Krizanauskiene A, Valkiūnas G, Bensch S (2007) Diversity and phylogeny of mitochondrial cytochrome B lineages from six morphospecies of avian Haemoproteus (Haemosporida: Haemoproteidae). J Parasitol 93:889–896. doi:10.1645/GE-1051R1.1
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon spp., Plasmodium spp. and Haemoproteus spp. from avian blood. J Parasitol 90:797–802. doi:10.1645/GE-184R1
Higgs S, Beaty BJ (2004) Natural cycles of vector-borne pathogens. In: Marquardt WC, Black WC, Freier JE, Hagedorn HH, Hemingway J, Higgs S, James AA, Kondratieff B, Moore CG (eds) Biology of disease vectors, 2nd edn. Elsevier Academic, San Diego, pp 167–186
Howe L, Castro IC, Schoener ER, Hunter S, Barraclough RK, Alley MR (2012) Malaria parasites (Plasmodium spp.) infecting introduced, native and endemic New Zealand birds. Parasitol Res 110:913–923. doi:10.1007/s00436-011-2577-z
Inci A, Yildirim A, Njabo KY, Duzlu O, Biskin Z, Ciloglu A (2012) Detection and molecular characterization of avian Plasmodium from mosquitoes in central Turkey. Vet Parasitol 188:179–184. doi:10.1016/j.vetpar.2012.02.012
Kim KS, Tsuda Y (2010) Seasonal changes in the feeding pattern of Culex pipiens pallens govern the transmission dynamics of multiple lineages of avian malaria parasites in Japanese wild bird community. Mol Ecol 19:5545–5554. doi:10.1111/j.1365-294X.2010.04897.x
Kimura M, Darbro JM, Harrington LC (2010) Avian malaria parasites share congeneric mosquito vectors. J Parasitol 96:144–151. doi:10.1645/GE-2060.1
Lacorte GA, Felix GM, Pinheiro RR, Chaves AV, Almeida-Neto G, Neves FS, Leite LO, Santos FR, Braga EM (2013) Exploring the diversity and distribution of neotropical avian malaria parasites—a molecular survey from southeast Brazil. PLoS ONE 8:e57770. doi:10.1371/journal.pone.0057770
Landau I, Chavatte JM, Peters W, Chabaud A (2010) The sub-genera of avian Plasmodium. Parasite 17:3–7. doi:10.1051/parasite/2010171003
LaPointe DA, Goff ML, Atkinson CT (2010) Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai’i. J Parasitol 96:318–324. doi:10.1645/GE-2290.1
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi:10.1093/bioinformatics/btm404
Leica Microsystems Switzerland Limited (2012) Software Leica Application Suite, LAS EZ Version 2.1.0. http://www.leica-microsystems.com/products/microscope-software/educational/details/product/leica-las-ez/downloads/. Accessed 30 Sep 2012
Loiseau C, Harrigan RJ, Robert A, Bowie RC, Thomassen HA, Smith TB, Sehgal RN (2012) Host and habitat specialization of avian malaria in Africa. Mol Ecol 21:431–441. doi:10.1111/j.1365-294X.2011.05341.x
Lotta IA, Matta NE, Torres RD, Moreno de Sandino M, Moncada LI (2013) Leucocytozoon fringillinarum and Leucocytozoon dubreuili in Turdus fuscater from a Colombian Páramo ecosystem. J Parasitol 99:359–362. doi:10.1645/GE-3156.1
Mantilla JS, Matta NE, Pacheco MA, Escalante AA, Gonzalez AD, Moncada LI (2013) Identification of Plasmodium (Haemamoeba) lutzi (Lucena, 1939) from Turdus fuscater (Great Thrush) in Colombia. J Parasitol 99:662–668. doi:10.1645/12-138.1
Martinsen ES, Paperna I, Schall JJ (2006) Morphological versus molecular identification of avian Haemosporidia: an exploration of three species concepts. Parasitology 133:279–288. doi:10.1017/S0031182006000424
Martinsen ES, Perkins SL, Schall JJ (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273. doi:10.1016/j.ympev.2007.11.012
Merino S, Moreno J, Vásquez RA, Martínez J, Sánchez-Monsálvez I, Estades CF, Ippi S, Sabat P, Rozzi R, McGehee S (2008) Haematozoa in forest birds from southern Chile: latitudinal gradients in prevalence and parasite lineage richness. Austral Ecol 33:329–340. doi:10.1111/j.1442-9993.2008.01820.x
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proc Gatew Comput Environ Workshop (GCE) 1–8. doi: 10.1109/gce.2010.5676129
Molina LF, Osorio J, Uribe E (1997) Cerros, humedales y areas rurales: Santa Fe de Bogota. DAMA, Bogota
Møller AP, Erritzøe J, Karadas F (2010) Levels of antioxidants in rural and urban birds and their consequences. Oecologia 163:35–45. doi:10.1007/s00442-009-1525-4
Pacheco MA, Escalante AA, Garner MM, Bradley GA, Aguilar RF (2011) Haemosporidian infection in captive masked bobwhite quail (Colinus virginianus ridgwayi), an endangered subspecies of the northern bobwhite quail. Vet Parasitol 182:113–120. doi:10.1016/j.vetpar.2011.06.006
Rambaut A (2006) FigTree: tree figure drawing too, version1.3.1. Institute of Evolutionary Biology, University of Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 12 May 2013
Rambaut A, Drummond AJ (2007) Tracer v1.5. http://tree.bio.ed.ac.uk/software/tracer/. Accessed 12 May 2013
Remsen JV, Cadena CD, Jaramillo A, Nores M, Pacheco JF, Pérez-Emán J, Robbins MB, Stiles FG, Stotz DF, Zimmer KJ (2012) A classification of the bird species of South America. American Ornithologists’ Union, version 7 December 2012. http://www.museum.lsu.edu/~Remsen/SACCBaseline.html. Accessed 7 Dec 2012
Ricklefs RE, Fallon SM (2002) Diversification and host switching in avian malaria parasites. Proc R Soc Lond B Biol Sci 269:885–892. doi:10.1098/rspb.2001.1940
Ricklefs RE, Outlaw DC (2010) A molecular clock for malaria parasites. Science 329:226–229. doi:10.1126/science.1188954
Ridgely RS, Allnutt TF, Brooks T, McNicol DK, Mehlman DW, Young BE, Zook JR, International Birdlife (2012) Digital distribution maps of the birds of the western hemisphere, version 5.0. In BirdLife International and NatureServe. Bird species distribution maps of the world. Turdus fuscater. In: IUCN Red List of Threatened Species. Version 2012.2. http://maps.iucnredlist.org/map.html?id=106006415. Accessed 12 May 2013
Rodríguez OA, Moya H, Matta NE (2009) Avian blood parasites in the National Natural Park Chingaza: high Andes of Colombia. Hornero 24:1–6
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Salazar MJ, Moncada LI (2004) Life cycle of Culex quinquefasciatus Say (Diptera: Culicidae) under uncontrolled conditions. Biomedica 24:385–392
Sambrook J, Fritsch EF, Maniatis T (1989) Chapter 6 Preparation and analysis of eukaryotic genomic DNA. In: Molecular cloning a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, New York, pp 6.4–6.12
Santiago-Alarcon D, Palinauskas V, Schaefer HM (2012) Diptera vectors of avian haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol Rev Camb Philos Soc 87:928–964. doi:10.1111/j.1469-185X.2012.00234.x
Schmidt KA, Ostfeld RS (2001) Biodiversity and the dilution effect in disease ecology. Ecology 82:609–619. doi:10.1890/0012-9658(2001)082[0609:BATDEI]2.0.CO;2
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi:10.1038/nmeth.2089
Shahabuddin M, Costero A (2001) Spatial distribution of factors that determine sporogonic development of malaria parasites in mosquitoes. Insect Biochem Mol Biol 31:231–240
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC, Boca Raton
Valkiūnas G, Iezhova TA, Krizanauskiene A, Palinauskas V, Bensch S (2008a) In vitro hybridization of Haemoproteus spp.: an experimental approach for direct investigation of reproductive isolation of parasites. J Parasitol 94:1385–1394. doi:10.1645/GE-1569.1
Valkiūnas G, Iezhova TA, Loiseau C, Smith TB, Sehgal RN (2009) New malaria parasites of the subgenus Novyella in African rainforest birds, with remarks on their high prevalence, classification and diagnostics. Parasitol Res 104:1061–1077. doi:10.1007/s00436-008-1289-5
Valkiūnas G, Zehtindjiev P, Dimitrov D, Krizanauskiene A, Iezhova TA, Bensch S (2008b) Polymerase chain reaction-based identification of Plasmodium (Huffia) elongatum, with remarks on species identity of haemosporidian lineages deposited in GenBank. Parasitol Res 102:1185–1193. doi:10.1007/s00436-008-0892-9
Zamora-Vilchis I, Williams SE, Johnson CN (2012) Environmental temperature affects prevalence of blood parasites of birds on an elevation gradient: implications for disease in a warming climate. PLoS ONE 7:e39208. doi:10.1371/journal.pone.0039208
Zehtindjiev P, Krizanauskiene A, Bensch S, Palinauskas V, Asghar M, Dimitrov D, Scebba S, Valkiūnas G (2012a) A new morphologically distinct avian malaria parasite that fails detection by established polymerase chain reaction-based protocols for amplification of the cytochrome B gene. J Parasitol 98:657–665. doi:10.1645/GE-3006.1
Zehtindjiev P, Križanauskienė A, Scebba S, Dimitrov D, Valkiūnas G, Hegemann A, Tieleman BI, Bensch S (2012b) Haemosporidian infections in skylarks (Alauda arvensis): a comparative PCR-based and microscopy study on the parasite diversity and prevalence in southern Italy and the Netherlands. Eur J Wildl Res 58:335–344. doi:10.1007/s10344-011-0586-y
Acknowledgments
This study was partially supported by a Project Management Program of the Department of Welfare of the Universidad Nacional de Colombia, Bogotá, project number UGP164. The authors wish to thank all the students belonging to the Host-Parasite Relationship Research Group: Avian Hemoparasites Model, especially to Ingrid Lotta for assistance to obtain the sequences. We thank A. Warren, the Natural History Museum, London, UK, for providing the type and voucher material of P. vaughani. Dr. Tatjana Iezhova is acknowledged for assistance during preparation of plates of the illustrations and Dr. C. E. Beard for his comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mantilla, J.S., González, A.D., Valkiūnas, G. et al. Description and molecular characterization of Plasmodium (Novyella) unalis sp. nov. from the Great Thrush (Turdus fuscater) in highland of Colombia. Parasitol Res 112, 4193–4204 (2013). https://doi.org/10.1007/s00436-013-3611-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3611-0