Abstract
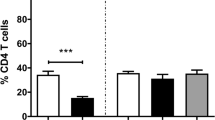
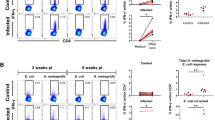
Eimeria bovis infections commonly have clinical impact only on young animals, as homologous reinfections generally are under immunological control. So far, the nature of the immune responses delivering protection to calves has not been investigated. In this study we therefore analysed local and peripheral proliferative T cell activities of primary- and challenge-infected calves and investigated the occurrence of T cell phenotypes in the peripheral blood and in mucosal gut segments isolated either by bioptic means or by necropsies. We show that lymphocytes of E. bovis-infected calves exhibit effective, transient antigen-specific proliferative responses in the course of prepatency of primary infection but fail to react after homologous reinfection suggesting early abrogation of parasite development. Whilst in primary infection an expansion of peripheral CD4+ T cells was observed, reinfection had no effect on the proportions of CD4+, CD8+ subsets or γδTCR+ T cells. In contrast, both E. bovis primary and challenge infections had an impact on local tissue T cell distribution. Primary infection was characterised by a CD4+ T cell infiltration early in prepatency in ileum and later in colon mucosa, whereas CD8+ T cells were only found accumulating in the latter gut segment. Challenge infection led to infiltration of both CD4+ and CD8+ T cells in small intestine and large intestine segments indicating protective functions of both cell types. In contrast, infiltration of ileum and colon mucosa with γδTCR+ T cells was restricted to primary infection.







Similar content being viewed by others
References
Badawy AI, Lutz K, Taubert A, Zahner H, Hermosilla C (2010) Eimeria bovis meront I-carrying host cells express parasite-specific antigens on their surface membrane. Vet Res Commun, doi:10.1007/s11259-009-9336-y
Behling-Kelly E, Czuprynski CJ (2007) Endothelial cells as active participants in veterinary infections and inflammatory disorders. Anim Health Res Rev 8:47–58
Bosse D, George V, Candal FJ, Lawley TJ, Ades EW (1993) Antigen presentation by a continuous human microvascular endothelial cell line, HMEC-1, to human T cells. Pathobiology 61:236–238
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breed DG, Dorrestein J, Vermeulen AN (1996) Immunity to Eimeria tenella in chickens: phenotypical and functional changes in peripheral blood T-cell subsets. Avian Dis 40:37–48
Daugschies A, Najdrowski M (2005) Eimeriosis in cattle: current understanding. J Vet Med B Infect Dis Vet Public Health 52:417–427
Daugschies A, Bürger HJ, Akimaru M (1998) Apparent digestibility of nutrients and nitrogen balance during experimental infection of calves with Eimeria bovis. Vet Parasitol 77:93–102
De Libero G (1997) Sentinel function of broadly reactive human gamma delta T cells. Immunol Today 18:22–26
Denkers EY, Sher A, Gazzinelli RT (1993) CD8+ T-cell interactions with Toxoplasma gondii: implications for processing of antigen for class-I-restricted recognition. Res Immunol 144:51–57
Epperson DE, Pober JS (1994) Antigen-presenting function of human endothelial cells. Direct activation of resting CD8 T cells. J Immunol 153:5402–5412
Fiege N, Klatte D, Kollmann D, Zahner H, Bürger HJ (1992) Eimeria bovis in cattle: colostral transfer of antibodies and immune response to experimental infections. Parasitol Res 78:32–38
Findly RC, Roberts SJ, Hayday AC (1993) Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur J Immunol 23:2557–2564
Fitz-Coy SH (1992) Antigenic variation among strains of Eimeria maxima and E. tenella of the chicken. Avian Dis 36:40–43
Fitzgerald PR (1980) The economic impact of coccidiosis in domestic animals. Adv Vet Sci Comp Med 24:121–143
Friend SC, Stockdale PH (1980) Experimental Eimeria bovis infection in calves: a histopathological study. Can J Comp Med 44:129–140
Hermosilla C, Bürger HJ, Zahner H (1999) T cell responses in calves to a primary Eimeria bovis infection: phenotypical and functional changes. Vet Parasitol 84:49–64
Hermosilla C, Barbisch B, Heise A, Kowalik S, Zahner H (2002) Development of Eimeria bovis in vitro: suitability of several bovine, human and porcine endothelial cell lines, bovine fetal gastrointestinal, Madin-Darby bovine kidney (MDBK) and African green monkey kidney (VERO) cells. Parasitol Res 88:301–307
Hughes HP, Thomas KR, Speer CA (1988) Antigen-specific lymphocyte transformation induced by oocyst antigens of Eimeria bovis. Infect Immun 56:1518–1525
Hughes HP, Whitmire WM, Speer CA (1989) Immunity patterns during acute infection by Eimeria bovis. J Parasitol 75:86–91
Jackson AR (1964) The isolation of variable coccidial sporozoites. Parasitology 54:87–93
Kasper LH, Khan IA, Ely KH, Buelow R, Boothroyd JC (1992) Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J Immunol 148:1493–1498
Khan IA, Kasper LH (1996) IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol 157:2103–2108
Knolle PA (2006) Cognate interaction between endothelial cells and T cells. Results Probl Cell Differ 43:151–173
Lillehoj HS (1994) Analysis of Eimeria acervulina-induced changes in the intestinal T lymphocyte subpopulations in two chicken strains showing different levels of susceptibility to coccidiosis. Res Vet Sci 56:1–7
Martin AG, Danforth HD, Barta JR, Fernando MA (1997) Analysis of immunological cross-protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of Eimeria maxima. Int J Parasitol 27:527–533
Norton CC, Hein HE (1976) Eimeria maxima: a comparison of two laboratory strains with a fresh isolate. Parasitology 72:345–354
Ovington KS, Alleva LM, Kerr EA (1995) Cytokines and immunological control of Eimeria spp. Int J Parasitol 25:1331–1351
Pober JS, Cotran RS (1991) Immunologic interactions of T lymphocytes with vascular endothelium. Adv Immunol 50:261–302
Pollock JM, Welsh MD (2002) The WC1(+) gammadelta T-cell population in cattle: a possible role in resistance to intracellular infection. Vet Immunol Immunopathol 89:105–114
Renaux S, Quere P, Buzoni-Gatel D, Sewald B, Le VY, Coudert P, Drouet-Viard F (2003) Dynamics and responsiveness of T-lymphocytes in secondary lymphoid organs of rabbits developing immunity to Eimeria intestinalis. Vet Parasitol 110:181–195
Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC (1996) T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A 93:11774–11779
Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ (2003) Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol 33:3117–3126
Rose ME (1973) Immunity. In: Hammond DM, Long PL (eds) The coccidia. University Park Press and Butterworth, Baltimore, pp 295–341
Rose ME (1987) Immunity to Eimeria infections. Vet Immunol Immunopathol 17:333–343
Rose ME, Hesketh P, Wakelin D (1992a) Immune control of murine coccidiosis: CD4+ and CD8+ T lymphocytes contribute differentially in resistance to primary and secondary infections. Parasitology 105(Pt 3):349–354
Rose ME, Millard BJ, Hesketh P (1992b) Intestinal changes associated with expression of immunity to challenge with Eimeria vermiformis. Infect Immun 60:5283–5290
Rose ME, Hesketh P, Rothwell L, Gramzinski RA (1996) T-cell receptor gamma–delta lymphocytes and Eimeria vermiformis infection. Infect Immun 64:4854–4858
Rothwell L, Gramzinski RA, Rose ME, Kaiser P (1995) Avian coccidiosis: changes in intestinal lymphocyte populations associated with the development of immunity to Eimeria maxima. Parasite Immunol 17:525–533
Schito ML, Chobotar B, Barta JR (1998a) Major histocompatibility complex class I- and II-deficient knock-out mice are resistant to primary but susceptible to secondary Eimeria papillata infections. Parasitol Res 84:394–398
Schito ML, Chobotar B, Barta JR (1998b) Cellular dynamics and cytokine responses in BALB/c mice infected with Eimeria papillata during primary and secondary infections. J Parasitol 84:328–337
Shi M, Huther S, Burkhardt E, Zahner H (2001) Lymphocyte subpopulations in the caecum mucosa of rats after infections with Eimeria separata: early responses in naive and immune animals to primary and challenge infections. Int J Parasitol 31:49–55
Shirley MW, Bellatti MA (1988) Live attenuated coccidiosis vaccine: selection of a second precocious line of Eimeria maxima. Res Vet Sci 44:25–28
Smith AL, Hayday AC (1998) Genetic analysis of the essential components of the immunoprotective response to infection with Eimeria vermiformis. Int J Parasitol 28:1061–1069
Smith AL, Hayday AC (2000a) An alphabeta T-cell-independent immunoprotective response towards gut coccidia is supported by gammadelta cells. Immunology 101:325–332
Smith AL, Hayday AC (2000b) Genetic dissection of primary and secondary responses to a widespread natural pathogen of the gut, Eimeria vermiformis. Infect Immun 68:6273–6280
Smith AL, Hesketh P, Archer A, Shirley MW (2002) Antigenic diversity in Eimeria maxima and the influence of host genetics and immunization schedule on cross-protective immunity. Infect Immun 70:2472–2479
Staska LM, McGuire TC, Davies CJ, Lewin HA, Baszler TV (2003) Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect Immun 71:3272–3279
Stockdale PH (1977) The pathogenesis of the lesions produced by Eimeria zuernii in calves. Can J Comp Med 41:338–344
Taubert A, Zahner H, Hermosilla C (2006) Dynamics of transcription of immunomodulatory genes in endothelial cells infected with different coccidian parasites. Vet Parasitol 142:214–222
Taubert A, Hermosilla C, Sühwold A, Zahner H (2008) Antigen-induced cytokine production in lymphocytes of Eimeria bovis primary and challenge infected calves. Vet Immunol Immunopathol 126:309–320
Trout JM, Lillehoj HS (1995) Eimeria acervulina infection: evidence for the involvement of CD8+ T lymphocytes in sporozoite transport and host protection. Poult Sci 74:1117–1125
Trout JM, Lillehoj HS (1996) T lymphocyte roles during Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol 53:163–172
Vervelde L, Vermeulen AN, Jeurissen SH (1996) In situ characterization of leucocyte subpopulations after infection with Eimeria tenella in chickens. Parasite Immunol 18:247–256
Wagner CR, Vetto RM, Burger DR (1984) The mechanism of antigen presentation by endothelial cells. Immunobiology 168:453–469
Wakelin D, Rose ME (1990) Immunity to coccidiosis. In: Long PL (ed) Coccidiosis of man and domestic animals. CRC Press, Florida, pp 281–306
Wakelin D, Rose ME, Hesketh P, Else KJ, Grencis RK (1993) Immunity to coccidiosis: genetic influences on lymphocyte and cytokine responses to infection with Eimeria vermiformis in inbred mice. Parasite Immunol 15:11–19
Wilson RA, Zolnai A, Rudas P, Frenyo LV (1996) T-cell subsets in blood and lymphoid tissues obtained from fetal calves, maturing calves, and adult bovine. Vet Immunol Immunopathol 53:49–60
Zahner H, Homrighausen-Riester C, Bürger HJ (1994) Eimeriosen. In: Röllinghoff M, Rommel M (eds) Immunologische und molekulare Parasitologie. Gustav Fischer Verlag, Jena, Stuttgard, pp 67–82
Acknowledgements
We acknowledge B. Hofmann and C. Scheld for their excellent technical assistance in cell culture. We also thank K. Failing (Giessen) for support in statistical analyses of the data. This work was supported by the German Research Foundation (DFG; project Za 67/6-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sühwold, A., Hermosilla, C., Seeger, T. et al. T cell reactions of Eimeria bovis primary- and challenge-infected calves. Parasitol Res 106, 595–605 (2010). https://doi.org/10.1007/s00436-009-1705-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1705-5