Abstract
The Brazilian Pantanal has been considered one of the richest and most diverse wetland ecosystems in the world. It is occupied by cattle ranching, and a variety of wildlife species share the same habitats with domestic livestock. We investigated infections of Trypanosoma evansi and Trypanosoma cruzi in the sympatric suiformes-collared peccary (Tayassu tajacu), white-lipped peccary (Tayassu pecari), and feral pig (Sus scrofa) by parasitological, serological, and molecular tests. Additionally, we evaluated the health status of both positive and negative suiformes by hematological and biochemical parameters. The results show that peccaries and feral pigs play an important role on the maintenance of both T. evansi and T. cruzi in the Brazilian Pantanal. Health impairment was observed only in the white-lipped peccary infected with T. evansi. Despite presenting low T. evansi parasitemia, all infected white-lipped peccaries displayed low hematocrit values and marked leucopenia. The hematological values showed that the T. evansi infection is more severe in young white-lipped peccaries. The presented data show that feral pigs and peccaries are immersed in the transmission net of both trypanosome species, T. cruzi and T. evansi, in the Pantanal region.
Similar content being viewed by others
Introduction
Parasites are common in free-living animals and play a decisive role in the structures of host communities, acting in the process of natural selection, extinction, and generation of new species. In addition, the pattern of parasitic infection changes according to host abundance and environmental conditions. Consequently, hosts are linked with their parasites in a dynamic equilibrium that, if ruptured, may result in disease outbreaks. The appearance of emerging and reemerging diseases worldwide demonstrates that the complexity of the triad host–parasite–environment is still underestimated in that many of the details concerning the parasitic transmission cycle continue to be unknown. This is specially the case of trypanosomiasis that in Latin America is due mainly to Trypanosoma evansi and Trypanosoma cruzi, both of which infect a broad range of mammal species. However, almost nothing is known about these trypanosomatid species in feral pigs and peccaries throughout the Pantanal region, Brazil.
The Pantanal wetland is a seasonally flooded basin covering approximately 140,000 km2 in the core of South America. This region constitutes an important agro-industry center in Latin America, supporting a cattle population of around 4.5 million (Mourão et al. 2002). Wildlife is abundant in this region and shares the same habitat with livestock (Lourival and Fonseca 1998). Despite increased human encroachment, the Pantanal ecosystem is considered to be one of the most well-preserved biomes in Brazil and was added to United Nations Educational, Scientific, and Cultural Organization’s World Heritage List in 2000. Moreover, the Brazilian Pantanal has been described as a “biological hotspot” for conservation, one of the ten most biologically diverse regions, worldwide (Myers et al. 2000). In spite of the importance of this region for the Brazilian economy and biodiversity, the transmission cycles of parasites that cross-infect humans, domestic animals, and wildlife in the Pantanal region is still limited.
Although phenotypically similar to pigs and belonging to the same order Artiodactyla, the collared peccary (Tayassu tajacu) and the white-lipped peccary (WLP; Tayassu pecari) belong to the Tayassuidae family and the feral pig (Sus scrofa) to the Suidae family. Following the early divergence from a common ancestor during the late Eocene–early Oligocene, peccaries and pigs evolved separately: peccaries in North America and pigs in Eurasia (Ducrocq 1994). Today, the geographic range of the gregarious peccaries extends from the southwestern US to Northern Uruguay (Sowls 1984). At the study site, the population densities for WLP and collared peccaries were estimated around 9.6 and 3.7 individuals per square kilometer, respectively (Desbiez 2007). Feral pigs, which were found in the Pantanal since the 1800s, are considered one of the “world’s worst invasive alien species” by the World Conservation Union (IUCN 2000). The population of feral pigs in the Brazilian Pantanal has been estimated to be over one million animals dispersed in 10,000 groups (Mourão et al. 2002).
The protozoans, T. cruzi and T. evansi (Kinetoplastida, Trypanosomatidae), are considered to be of medical importance because these species are the etiological agents of the arthropod-borne Chagas disease (American trypanosomiasis) and “Mal de Cadeiras” (Surra), respectively. These flagellates display strikingly distinct life strategies and are known to have a large diversity of domestic and wild mammalian hosts. Besides vectorial transmission, hosts also may become infected by both T. cruzi and T. evansi through the oral route. It is worth mentioning that the more important pathological feature due to T. cruzi infection refers to cardiopathy, while the main outcome of T. evansi infection is severe anemia. Both flagellates have been found in the Pantanal region infecting dozens of host species (Herrera et al. 2004, 2005, 2007).
The ecological scenario that favors the entrance of a new or alternative host in the transmission cycle of a given parasite remains poorly understood. Unraveling this puzzle can be crucial for wildlife conservation. In this context, the role played by peccaries and feral pigs on the transmission cycles of both T. evansi and T. cruzi in the Pantanal region, a sylvatic enzootic area for pathogenic trypanosomatids, is still largely unknown.
The aim of the current study was to: (a) estimate the prevalence of T. evansi and T. cruzi on peccaries and feral pigs in the Pantanal region; (b) evaluate the effect of these infections on the health status, expressed by hematology and serum biochemistry; and (c) review the role of the collared peccary, white-lipped peccary, and feral pigs in the transmission cycles of these flagellates.
Material and methods
Study area
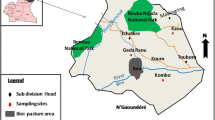
We sampled peccaries and feral pigs in the Rio Negro region, a well-preserved area of the southern Pantanal. The region is dominated by large areas of gallery forests and savannas, open grasslands, and thousands of shallow lake basins. Once a traditional ranching property, only 10% of the study site is used for cattle today; the remaining 90% has been transformed into a private reserve (5,400 ha). The length and severity of flooding and drought vary from year to year.
Collection of samples
We collected blood from WLP, collared peccaries, and feral pigs during 2002 and 2003. Animals were baited with corn and manioc and caught by trapping. Traps consisted of boxes (3 × 1.5 × 1.5 m) with a guillotine-style drop gate door triggered by a rope in the rear of the trap. All trapping and handling procedures were conducted in accordance with the authorization of the Brazilian Environment Protection Institute. In addition, biosafety techniques and individual protection equipment were used for all sampling procedures.
Peccaries and feral pigs were immobilized with zolazepam and tiletamine chlorhydrate (Zoletil® 50), sexed, weighed, and placed in rough age classes based on tooth wear (Sowls 1984). Blood was collected by vein puncture using a vacuum system (Vacuum II®, Labnew, Campinas, Brazil) containing ethylenediaminetetraacetic acid. The animal was then placed back into the trap and was not released until it was fully recovered from the anesthetic. For biochemical analyses and serological tests, plasma and sera were separated by centrifugation and stored at −20°C until use.
Parasite infection
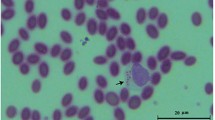
The microhematocrit centrifuge technique (MHCT) was used to search for trypomastigote blood forms of T. cruzi and T. evansi (Woo 1970). Hemoculture (HC) was performed for T. cruzi in Novy, McNeal, and Nicolle medium covered with an overlay of liver infusion tryptose mixed with 10% fetal calf serum and 140 mg/ml of gentamicin sulfate. The tubes were examined in the laboratory twice monthly up to 5 months. High parasitemia was defined by positive MHCT for both trypanosomatids and in the case of T. cruzi also by HC.
In order to obtain DNA for carrying out the molecular test polymerase chain reaction (PCR) for T. evansi, 30 μl of whole blood was applied on filter paper confetti placed in a 0.5-ml Eppendorf tube, dried, and stored at room temperature.
DNA extraction from the buffy coat collected on filter paper and PCR diagnoses were performed according to procedures previously described (Herrera et al. 2005).
We used the immunofluorescence antibody test to detect antibodies against T. cruzi and T. evansi according to standard procedures (Camargo 1964), with minor modifications (Herrera et al. 2004). Antigen used in T. cruzi serology was obtained from parasites harvested from axenic culture in the exponential phase. For T. evansi, antigen was prepared by filtration of rodent infected blood through anion exchange chromatography column (diethylaminoethyl cellulose) as described by Lanham and Godfrey (1970). Parasites were washed three times by centrifugation with 0.1 M phosphate buffered saline pH 7.4 and resuspended in 0.85% saline solution. The suspension containing parasites was diluted to yield 40 cells per field under ×400 magnification and distributed on glass slides. Negative control samples were obtained from animals born at a zoo and positive control serum samples were obtained from positive parasitologically testing animals (including PCR). We used the antibody conjugate antipig immunoglobulin G (fluorescein isothiocyanate, Sigma®) for the three suiformes species. The cutoff values defined for T. cruzi and T. evansi were 1:10 since it was the lowest serum sample titer in which parasites could be detected by both HC and PCR.
Hematology
The following tests were performed during the first 12 h after blood collection: (1) packed cell volume (PCV), measured by the MHCT; (2) total red blood cells (RBC) and total white blood cells counted in a Neubauer chamber; and (3) hemoglobin (Hb) concentration, determined using a spectrophotometer. Red cell indexes such as the mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were calculated from the results obtained from RBC, Hb, and PCV. We performed the differential leukocyte count using blood smears fixed with methanol and stained with Giemsa.
Biochemical assays
In the same week of collection, we measured: total protein, albumin, bilirubin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and the plasmatic determination of fructose in the plasma samples using enzymatic colorimetric methods, by commercial kits. The globulin values were obtained from the difference between total protein and albumin concentration. Statistical analyses for biochemical assays were only performed for WLP.
Statistical analyses
We conducted Chi-square (X 2) and Kruskal–Wallis tests to determine differences among hematological and biochemical values by age and sex groups. The Pearson analysis was applied for evaluating the correlation between hematological and biochemical values from positive and negative WLP samples. We used the package Statistical Analysis System (Stokes et al. 2000) only for WLP data since the number of captured animals of the other examined swine species was too low.
Results
Animals
We sampled 68 WLP, 39 adults (ten males and 29 females), 20 subadults (11 males and nine females), and nine young (four males and five females), eight adult collared peccaries (six males and two females), and 15 adult feral pigs (12 males and nine females). All animals examined were in good physical condition and clinically normal.
Parasite infections
All three suiformes species displayed high T. evansi serum prevalence with low parasitemias detected only by PCR. High serum prevalence of T. cruzi infection was recorded in the three species sampled. However, high parasitemias as corroborated by positive HC were only found in feral pigs. The prevalence of parasite infections in peccaries and feral pigs are shown in Table 1. No mixed infection by T. cruzi and T. evansi could be detected with any of the employed tests.
Hematological and biochemical analyses
Only T. evansi was associated with a health deficit concern in WLP. In this sense, we found the following significant hematological differences: (1) reduced RBC (p < 0.02), platelet (p < 0.05), and lymphocyte counts (p < 0.04) in infected young; (2) decrease in leucocyte counts and hematocrit values for all infected animals of the three age groups (p < 0.05); (3) increase in neutrophil counts (p < 0.005) for infected young WLP. The means and standard deviations of hematological values that displayed statistical difference between T.-evansi-infected and uninfected WLP are shown in Table 2.
Discussion
A reservoir host is best defined as one or a complex of species responsible for the long-term maintenance of a given parasite into a particular environment. Thus, the host–parasite–environment constitutes a peculiar biologic unit that changes continuously (Ashford 1996).
WLP infected by T. evansi displayed anemia as a cardinal sign, a common feature found in all other hosts species, except for capybaras (Hydrochoerus hydrochaeris; Franke et al. 1994; Herrera et al. 2004). The anemia observed in all WLP infected by T. evansi was microcytic normochromic, as a result of the significantly lower hematocrit values. Anemia was more expressive in infected young WLP. The mechanisms underlying anemia in T.-evansi-infected animals are still under debate. Hemolysis as a result of immune mediated erythrophagocytosis and depression of erythropoiesis may be implicated (Seed and Hall 1985; Silva et al. 1995).
The significant decrease of the leukocyte counts found in all T.-evansi-infected WLP suggests an immune suppression effect (Holland et al. 2003). The probable consequence of such suppression is that animals become more susceptible to secondary infection. Mainly in infected young WLP, the registered marked decrease of lymphocyte counts is a significant complicating factor. In addition, the intense neutrophilia recorded in young infected WLP may be related to the recurrent peaks of T. evansi parasitemia, a common feature in salivarian trypanosomes (Woo 1970; Silva et al. 1995).
T. evansi infection in juvenile animals is more severe due to the decrease of the platelet count that may be associated to a disseminated intravascular coagulation (DIC), an important homeostatic disorder found in T. evansi infections (Sudarto et al. 1990; Delarue et al. 1997). The genesis of DIC is associated with the deposit of immune complexes in the microcirculation, leading to a pathological activation of the coagulation system. Also, these immune complexes are known to cause damage in the heart, liver, brain, and kidneys (Robinson and Maxie 1993).
The data show that peccaries and feral pigs in the Pantanal region act as a maintenance host for T. evansi in the natural environment due to their cryptic infections (only detectable by PCR) associated with high serum prevalence rates. Apparently, pigs efficiently control infections by T. evansi since both experimental and natural infections resulted in low parasitemias (Reid et al. 1999; Ng’ayo et al. 2005). Periods of low or undetectable parasitemia do not assure vectorial transmission but may maintain the long-lasting course of infection (Herrera et al. 2001). As rapid onsets of T. evansi parasitemia can occur due to stress conditions, such as prolonged droughts and habitat loss, these gregarious species may also act as competent reservoirs, assuring the vectorial transmission of T. evansi in this biome.
Feral pigs appeared to be implicated with the maintenance of T. cruzi in the wild and may constitute a human health risk due to: (1) having a high T. cruzi serum prevalence rate, with high parasitemias; (2) their abundance; (3) providing an important link between sylvatic and domestic transmission cycles by the invasion of peridomestic areas; and (4) local people getting into close contact with feral pigs through traditional hunting practices (Lourival and Fonseca 1998).
The abundance and diversity of ecological resources in the Brazilian Pantanal maintain a dynamic equilibrium among parasites, hosts, and the environment. This phenomenon is recognized as a buffer system for occurrence of outbreaks (Dobson et al. 2006). However, the Brazilian Pantanal has been suffering a continuous human interference via substitution of forested areas and native grassland by exotic pastures. The principal epidemiological outcome of this ecological simplification is the expansion of the interface between wildlife, humans, and domestic animals and consequent enhancement of pathogens flow, which may lead to disease emergence. In this context, the challenge of maintaining natural epidemiological balance while allowing economic growth is a major concern of health and conservation organizations.
References
Ashford RW (1996) Leishmaniosis reservoirs and their significance in control. Clin Dermatol 14:523–532
Camargo ME (1964) Improved technique of indirect immunofluorescence for serological diagnosis for toxoplasmosis. Rev Inst Med Trop São Paulo 3:117–118
Delarue ML, Silva RAS, De Carli GA (1997) Coagulopathy in dogs infected with Trypanosoma (Trypanozoon) evansi (STEEL, 1885) Balbiani, 1888. Parasitología al Día 21:3–4
Desbiez ALJ (2007) Wildlife conservation in the Pantanal: habitat alteration, invasive species and bushmeat hunting. Thesis, Ph.D., University of Kent at Canterbury, England.
Dobson A, Cattadori I, Holt RD, Ostfeld RS, Keesing F, Krichbaum K, Rohr JR, Perkins SE, Hudson PJ (2006) Sacred cows and sympathetic squirrels: the importance of biological diversity to human health. PLoS Med 3:e231
Ducrocq S (1994) An Eocene peccary from Thailand and the biogeographical origins of the artiodactyl family Tayassuidae. Palaeontology 37:765–779
Franke CR, Greiner M, Mehlitz D (1994) Monitoring of clinical, parasitological and serological parameters during an experimental infection of cabybaras (Hydrochoerus hydrochaeris) with Trypanosoma evansi. Acta Trop 58:171–174
Herrera HM, Aquino LPCT, Menezes RF, Marques LC, Moraes MAV, Werther K, Machado RZ (2001) Trypanosoma evansi experimental infection in South American coati (Nasua nasua): clinical, parasitological and humoral immune response. Vet Parasitol 102:209–216
Herrera HM, Dávila AMR, Norek A, Abreu UG, Souza SS, D’Andrea PS, Jansen AM (2004) Enzootiology of Trypanosoma evansi in Pantanal, Brazil. Vet Parasitol 125:263–275
Herrera HM, Norek A, Freitas TPT, Rademaker V, Fernandes O, Jansen AM (2005) Domestic and wild mammals infection by Trypanosoma evansi in a pristine area of the Brazilian Pantanal region. Parasitol Res 96:121–126
Herrera HM, Rademaker V, Abreu UG, D’Andrea PS, Jansen AM (2007) Variables that modulate the spatial distribution of Trypanosoma cruzi and Trypanosoma evansi in the Brazilian Pantanal. Acta Trop 102:55–62
Holland WG, Do TT, Huong NT, Dung NT, Thanh NG, Vercruysse J, Goddeeris BM (2003) The effect of Trypanosoma evansi infection on pig performance and vaccination against classical swine fever. Vet Parasitol 111:115–123
IUCN (2000) The IUCN guidelines for the prevention of biodiversity loss caused by alien invasive species. As approved by 51st Meeting of Council, http://app.iucn.org/biodiversityday/100booklet.pdf.
Lanham SM, Godfrey DG (1970) Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol 28:521–534
Lourival RFF, da Fonseca GAB (1998) Analise de Sustentabilidade do Modelo de Caça Tradicional, no Pantanal da Nhecolândia, Corumbá, MS. In: Valladares-Padua C, Bodmer RE, Cullen L Jr (eds) Manejo e Conservação de Vida Silvestre no Brasil. MCT-CNPq, Sociedade Civil Mamirauá, Brasília
Mourão GM, Coutinho ME, Mauro RA, Tomás WM, Magnusson W (2002) Levantamento aéreos de espécies introduzidas no Pantanal: porco ferais (porco monteiro), gado bovino e búfalos. Boletim de Pesquisa e Desenvolvimento, Embrapa Pantanal, Corumbá, 28, ISSN 1517-1981, http://www.cpap.embrapa.br/publicacoes/online/BP28
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kents J (2000) Biodiversity hotpots for conservation priorities. Nature 403:853–858
Ng’ayo MO, Njiru ZK, Kenya EU, Muluvi GM, Osir EO, Masiga DK (2005) Detection of trypanosomes in small ruminants and pigs in western Kenya: important reservoirs in the epidemiology of sleeping sickness? Kinet Biol Dis 14:4–5
Reid SA, Husein A, Hutchinson GW, Copeman DB (1999) A possible role of rusa deer (Cervus timorensis russa) and wild pigs in spread of Trypanosoma evansi from Indonesia to Papua New Guinea. Mem Ins Oswaldo Cruz 94:195–197
Robinson WF, Maxie MG (1993) The vascular system. In: Jubb KVF, Kennedy PC, Palmer N (eds) Pathology of domestical animals, 4th edition, vol. 3. Academic, London
Seed JR, Hall JE (1985) Pathophysiology of African trypanosomiasis. In: Tizard IR (ed) Immunology and pathogenesis of trypanosomiasis. CRC, Boca Raton
Silva RAMS, Herrera HM, Domingos LBS, Ximenes FA, Dávila AMR (1995) Pathogenesis of Trypanosoma evansi infection in dogs and horses: hematological and clinical aspects. Ciênc Rural 25:223–238
Sowls LK (1984) The peccaries. University of Arizona Press, Tucson
Stokes ME, Davis CS, Koch GG (2000) Categorical data analysis using SAS system, 2nd edn. SAS Institute Inc., Cary
Sudarto MW, Tabell H, Haines DM (1990) Immunohistochemical demonstration of Trypanosoma evansi in tissues of experimentally infected rats and a naturally infected water buffalo (Bubalus bubalis). J Parasitol 76:162–167
Woo PTK (1970) The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop 27:384–386
Acknowledgements
We are very grateful for the assistance provided by the local people and Dr. Vera Bongertz for English revision. This research was supported by funding from Conselho Nacional de Pesquisa e Desenvolvimento; Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do estado de Mato Grosso do Sul; Instituto Oswaldo Cruz/Fiocruz; Conservação Internacional do Brasil; Earthwatch Institute; and Fundação Manoel Barros.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrera, H.M., Abreu, U.G.P., Keuroghlian, A. et al. The role played by sympatric collared peccary (Tayassu tajacu), white-lipped peccary (Tayassu pecari), and feral pig (Sus scrofa) as maintenance hosts for Trypanosoma evansi and Trypanosoma cruzi in a sylvatic area of Brazil. Parasitol Res 103, 619–624 (2008). https://doi.org/10.1007/s00436-008-1021-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1021-5