Abstract
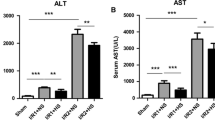
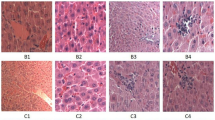
Membrane lipids and cytosolic proteins are major targets of oxidative injury. This study examined the effect of heat-shock preconditioning associated with the induction of heat-shock protein 72 on liver injury, from the aspect of lipid peroxidation and protein denaturation after carbon tetrachloride (CCl4) administration in rats — one of the representative oxidative injuries. Male Wistar rats were divided into two groups, group HS (preconditioned by heat exposure) and group C (not preconditioned). Expression of HSP72 in the liver tissue was confirmed by Western blot analysis. After a 48-h recovery period, all rats were given CCl4 intragastrically. Liver damage was assessed by measuring serum liver-related enzyme levels and adenine nucleotide concentration in the liver tissue. Lipid peroxidation and protein denaturation were evaluated by measuring tiobarbituric acid reactive substances (TBARS) and by immunohistochemical staining of 4-hydroxy-2-nonenal(HNE)-modified proteins in the liver. Survival rates of the rats after CCl4 administration were also compared. Expression of HSP72 was clearly detected in group HS, but not in group C. Heat-shock preconditioning significantly improved the survival rate, suppressed the increase in liver-related enzyme levels and maintained adenosine triphosphate levels (P<0.01 each). HNE-modified proteins — denatured proteins by free radical attack — were significantly less stained in group HS than in group C (P<0.05). However, TBARS levels did not differ between groups. Because heat-shock preconditioning did not alter TBARS levels but reduced HNE-modified proteins in association with the expression of HSP72, it is suggested that HSP72 did not prevent lipid peroxidation but decreased the lipid peroxidation-induced denaturation of proteins. This seemed to be a mechanism of heat-shock preconditioning to ameliorate oxidative liver injury.
Similar content being viewed by others
References
Atkinson DE (1968) The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 7: 4030–4034
Bagchi D, Carryl OR, Tran MX, Krohn RL, Bagchi DJ, Garg A, Bagchi M, Mitra S, Stohs SJ (1998) Stress, diet and alcohol-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. J Appl Toxicol 18: 3–13
Beckmann RP, Lovett M, Welch WJ (1992) Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J Cell Biol 117: 1137–1150
Bellmann K, Wenz A, Radons J, Burkart V, Kleemann R, Kolb H (1995) Heat-shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest 95: 2840–2845
Beyer RE (1983) A rapid biuret assay for protein of whole fatty tissues. Anal Biochem 129: 483–485
Chiang HL, Terlecky SR, Plant CP, Dice JF (1989) A role for a 70-kilodalton heat-shock protein in lysosomal degradation of intracellular proteins. Science 246: 382–385
Clawson GA (1989) Mechanisms of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res 8:104–112
Ewing JF, Haber SN, Maines MD (1992) Normal and heat-induced patterns of expression of heme oxygenase-1 (HSP32) in rat brain:hyperthermia causes rapid induction of mRNA and protein. J Neurochem 58:1140–1149
Ferrali M, Signorini C, Sugherini L, Pompella A, Lodovici M, Caciotti B, Ciccoli L, Comporti M (1997) Release of free, redox-active iron in the liver and DNA oxidative damage following phenylhydrazine intoxication. Biochem Pharmacol 53:1743–1751
Gaitanaris GA, Papavassiliou AG, Rubock P, Silverstein SJ, Gottesman ME (1990) Renaturation of denatured λ receptor requires heat-shock proteins. Cell 61: 1013–1020
Hightower LE (1991) Heat-shock, stress proteins, chaperones, and proteotoxicity. Cell 66: 191–197
Jaworek D, Gruber W, Bergmeyer HU (1974) Adenosine-5′-diphosphate and adenosine-5′-monophosphate. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York London, pp 2127–2131
Kajiwara I, Kawamura K, Hiratsuka Y, Takebayashi S (1996) The influence of oxygen free radical scavengers on the reduction of membrane-bound Na(+)-K(+)-ATPase activity induced by ischaemia/reperfusion injury in the canine kidney. Nephron 72: 637–643
Kamimura S, Tsukamoto H (1995) Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology 21: 1304–1309
Kim KB, Lee BM (1997) Oxidative stress to DNA, protein, and antioxidant enzymes (superoxide dismutase and catalase) in rats treated with benzo(a)pyrene. Cancer Lett 113: 205–212
Kume M, Yamamoto Y, Saad S, Gomi T, Kimoto S, Shimabukuro T, Yagi T, Nakagami M, Takada Y, Morimoto T, Yamaoka Y (1996) Ischemic preconditioning of the liver in rats:implications of heat-shock protein induction to increase tolerance of ischaemia-reperfusion injury. J Lab Clin Med 128: 251–258
Kuo JH, Chen HW, Chou RGR, Lii CK (1997) Vitamin E protection of cell morphology under oxidative stress is related to cytoskeletal proteins in rat hepatocytes. Arch Toxicol 71: 231–237
Lamprecht W, Trautschold I (1974) Adenosine-5′-triphosphate. Determination with hexokinase and glucose-6-phosphate dehydrogenase In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York London, pp 2101–2110
Liu SL, Esposti SD, Yao T, Diehl AM, Zern MA (1995) Vitamin E therapy of acute CCl4-induced hepatic injury in mice is associated with inhibition of nuclear factor kappa B binding. Hepatology 22: 1474–1481
Luna LG (1968) Manual of histological staining methods of the Armed Forces Institute of Pathology, 3rd edn. McGraw-Hill, New York, p 5
Luo XP, Yazdanpanah M, Bhooi N, Lehotay DC (1995) Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatographymass spectrometry. Anal Biochem 228: 294–298
Mason RP, Walter MF, Mason PE (1997) Effect of oxidative stress on membrane structure:small-angle X-ray diffraction analysis. Free Radic Biol Med 23: 419–425
Miyoshi H, Umeshita K, Sakon M, Imajohohmi S, Fujitani K, Gotoh M, Oiki E, Kambayashi J, Monden M (1996) Calpain activation in plasma membrane bleb formation during tertbutyl hydroperoxide-induced rat hepatocyte injury. Gastroenterology 110:1897–904
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by tiobarbituric acid reaction. Anal Biochem 95: 351–358
Remmer H, Kessler W, Einsele H, Hintze TH, Toranzo GDT, Gharaibeh AM, Frank H (1989) Ethanol promotes oxygen-radical attack on proteins but not on lipid. Drug Metab Rev 20:219–232
Rojas C, Cadenas S, Herrero A, Mendez J, Barja G (1996) Endotoxin depletes ascorbate in the guinea pig heart. Protective effects of vitamins C and E against oxidative stress. Life Sci 59:649–657
Skowyra D, Georgopoulos C, Zylicz M (1990) The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell 62:939–944
Stadtman ER (1992) Protein oxidation and aging. Science 257:1220–1224
Szweda LI, Uchida K, Tsai L, Stadtman ER (1993) Inactivation of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. J Biol Chem 268:3342–3347
Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H, Stadtman ER (1994) Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc Natl Acad Sci USA 91:2616–2620
Toyokuni S, Tanaka T, Hattori Y, Nishiyama Y, Yoshida A, Uchida K, Hiai H, Ochi H, Osawa T (1997) Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest 76: 365–374
Uchida K, Szweda LI, Chae HZ, Stadtman ER (1993) Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc Natl Acad Sci USA 90:8742–8746
Williams AT, Burk RF (1990) Carbon tetrachloride hepatotoxicity: an example of free radical-mediated injury. Semin Liver Dis 10:279–284
Yamashita N, Hoshida S, Nishida M, Igarashi J, Taniguchi N, Tada M, Kuzuya T, Hori M (1997) Heat-shock-induced manganese superoxide dismutase enhances the tolerance of cardiac myocytes to hypoxia-reoxygenation injury. J Mol Cell Cardiol 29:1805–1813
Zietara MS, Skorkowski EF (1995) Thermostability of lactate dehydrogenase LDH-A4 isoenzyme:Effect of heat-shock protein DnaK on the enzyme activity. Int J Biochem Cell Biol 27: 1169–1174
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported partly by a grant from the Scientific Research Fund of the Ministry of Education, Japan (no. 09307026 and no. 10557120) and partly by The Japan Society for the Promotion of Science, Japan (no. JSPS-RFTF96I00204).
Rights and permissions
About this article
Cite this article
Yamamoto, H., Yamamoto, Y., Yamagami, K. et al. Heat-shock preconditioning reduces oxidative protein denaturation and ameliorates liver injury by carbon tetrachloride in rats. Res. Exp. Med. 199, 309–318 (1999). https://doi.org/10.1007/s004339900040
Published:
Issue Date:
DOI: https://doi.org/10.1007/s004339900040