Abstract
Purpose
IKAROS family zinc finger 3 (IKZF3) is an oncogene involved in different malignancies, particularly in the development and malignant progression of lymphocytes. However, IKZF3 amplification and clinical significance in gastric cancers (GCs) remain unexplored.
Methods
We examined IKZF3 amplification status in 404 GCs with HER2 amplification status using tissue microarray (TMA) and fluorescence in situ hybridization (FISH) assays.
Results
IKZF3 amplification was detected in 6.9% (28/404) of all GC patients, with higher rates in intestinal-type gastric cancer (IGC) (11.22%, 22/196) compared to other types (2.88%, 6/208). HER2 amplification was identified in 16.09% (65/404) of all GC patients, with higher rates in IGC (20.92%, 41/196) compared to other types (11.54%, 24/208). Co-amplification of IKZF3 and HER2 was detected in 8.16% (16/196) of IGC patients and in 2.40% (5/208) of other types. IKZF3 amplification showed significant correlation with IGC (P = 0.001) and HER2 amplification (P = 0.0001). IKZF3 amplification exhibited significantly worse disease-free survival (DFS) (P = 0.014) and overall survival (OS) (P = 0.018) in GC patients, particularly in IGC (DFS: P < 0.001; OS: P < 0.001), rather than other types. Cox regression analysis demonstrate IKZF3 amplification as an independent poor prognostic factor in all GCs (P = 0.006, P = 0.004 respectively) and in IGC patients, regardless of stages I-II or III-IV (P = 0.007, P = 0.004 respectively). On the other hand, HER2 amplification was significantly associated with worse DFS (P = 0.008) and OS (P = 0.01) in IGC patients, but not in all GCs and in multivariate analysis. Within the subset of patients with HER2 amplification, those also exhibiting IKZF3 amplification displayed potential poorer prognosis (P = 0.08, P = 0.11 respectively).
Conclusion
IKZF3 amplification was detected in minority of GC patients, especially in IGC, and was an independent indicator of poor prognosis. Our study, for the first time, found the prognostic value of IKZF3 was superior to HER2 for GC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer, ranking fifth in terms of occurrence and second in global mortality caused by cancer, exhibits heterogeneous behaviors at multiple levels, including genetic mutations, tumor microenvironment, and cellular characteristics (Smyth et al. 2020). Approximately 50% of GC cases exist in east Asia (Liu et al. 2019). Various classifications were proposed to evaluate the histopathology of GC. Lauren’s classification system was proposed since 1965 (Lauren 1965), which is useful and widely used in GC to date. Generally, GC is classified into intestinal-type, diffuse-type and mixed-type (Aravind Sanjeevaiah 2018). Genetic alteration in different histologic types has been studied widely because of distinct pathological types, epidemiology, epigenetic aberrations and prognosis (Chia 2016). To date, surgical resection remains the major therapeutic strategy for current management of GC. Even though, the overall outcomes of GC still remain dismal because of the aggressive cancer behaviors and high recurrent rate (Chen et al. 2015; Song et al. 2017). The survival time of advanced GC patients is less than 12 months generally, and 5-year survival rate is less than 10% (Song et al. 2017). Besides, GC patients still bear huge medical costs, and there remains a great unmet need for treatment options to achieve better clinical outcomes.
In general, gastric cancer is highly heterogeneous in both clinical behaviors and molecular levels, our understandings of the changes of genetic landscape remains limited. Since the advent of targeted therapy, a game-changer in oncologic management in GC patients with HER2 amplification, for example, trastuzumab, many other related researches to explore the significance of gene amplification in GC rapidly intensified. Gene amplification generally refers to an increase in the number of gene copies in a specific region of a chromosome (Albertson 2006), exerting many biological effects on genome stability, cell proliferation, differentiation, invasion, metastasis, apoptosis and angiogenesis (Douglas H 2000), as well as being involved in drug resistance. Several amplified genes including ERBB2 (Kanayama et al. 2018; Nakata et al. 2019; Ughetto et al. 2021), FGFR2 (Kunii et al. 2008), MET (Hou et al. 2019), TNK2 (Shinmura et al. 2014) have been identified significantly associated with advanced tumor grade and heightened aggressiveness in gastric cancer. The investigation into the therapeutic potential of amplification in other oncogenes has been actively pursued in recent years. Even though, the understanding of numerous common amplifications in GC remains limited, which might be beneficial for oncology studies and development of related precision medicines (Lin et al. 2022). In our study, we investigated common gene amplification events in GC, which might be of clinical interest. We queried stomach cancer databases (TCGA, Nature 2014) deposited in cBioPortal (http://cbioportal.org), and the frequency of all amplified genes were ranked. An arbitrary cut-off of 10% frequency for gene amplification was used and IKZF3 was selected for further analysis in 14 genes, with the amplification frequency 11.3%. Our study confirmed IKZF3 amplification is commonly occurred in GC. IKZF3 belongs to the Ikaros family of zinc-finger proteins, which play an essential role in lymphatic differentiation, development and maintenance of homeostasis. There are five homologous members within the Ikaros transcription factor family, namely Ikaros (IKZF1), Helios (IKZF2), Aiolos (IKZF3), Eos (IKZF4), and Pegasus (IKZF5) (Heizmann et al. 2018).
IKZF3 expression in T-cells was reported as a predictive indicator for clinical outcomes in multiple myeloma patients (Awwad et al. 2018). Other researches showed mutant IKZF3 drives B cell neoplasia by modulating BCR and NF-κB signaling (Lazarian et al. 2021). Besides, it had been shown that high expression of IKZF3 usually indicate positive immunological responses and beneficial clinical results of skin cutaneous melanoma (Yang et al. 2022). IKZF3 has also been reported to have regulatory role in breast cancer, head and neck cancers, auto-immune diseases, and brain ischemia-reperfusion injury (Li et al. 2018, 2023; Lin et al. 2022; Meng et al. 2023). Very few studies have reported the significance of IKZF3 in GC. Here we investigate the potential clinical significance of IKZF3 in GC. Our data suggest that IKZF3 amplification often occurs in GC, and is predominantly altered in IGC. Our results revealed IKZF3 as a promising target in GC for both academic investigations and therapeutic development.
Methods
Patients selection
This study comprised a cohort of 404 patients who underwent surgical procedures for stomach lesions and were diagnosed with gastric cancer in Zhongshan Hospital, Fudan University, between January 2015 and January 2020. Patients who had received preoperative antitumor treatment, such as chemotherapy, neoadjuvant therapy and radiotherapy were excluded from this cohort.
All available hematoxylin and eosin (H&E) stained slides were independently reviewed and assessed by two senior pathologists (Hou YY, Xu C). The diagnostic criteria used for all cases were in accordance with the standard guidelines outlined in the fifth edition of the WHO classification of digestive system tumors, all cases were staged based on the rules specified within the eighth edition cancer staging manual of the American Joint Cancer Committee.
Clinicopathological characteristics including gender, age, lymph node metastasis (LNM), EBV infection, clinical stage and disease progression were obtained from related clinical records. Follow-up data of patients who did not revisit Zhongshan hospital for post-operative check were then interviewed by phone with patients and/or their families. Missed visit were exclude from this study.
Informed consent for the acquisition and use of tissue specimens and related clinical data of GC patients was obtained ahead of time from each patient. The Human Research Ethics Committee of Zhongshan Hospital, Fudan University has granted ethical approval for this study.
Tissue microarray preparation
The construction of tissue microarrays (TMAs) comprising tumor tissues of 404 GC patients was referred to the previous description (Yuan Shi 2013). In short, the representative regions of 2 × 6 mm with abundant tumor cells were chosen by two senior pathologists based on HE-staining results. The precise area on the formalin-fixed, paraffin-embedded (FFPE) tissue blocks were isolated subsequently, arrayed onto the recipient TMA blocks vertically and consolidated on precision instrument (Leica EG1150).
Fluorescence in situ hybridization
Dual-color FISH assay using a specific probe for IKZF3 or HER2 (Spectrum orange) in combination with a centromere-specific probe (Spectrum green) targeting chromosome 17 (CEP17) (Empire Genomics, Buffalo, NY) was administered on the TMA sections of 3 μm thickness based on standard operation procedure (Oshima et al. 2014) to evaluate IKZF3 or HER2 amplification. IKZF3 or HER2 amplification was confirmed by two senior pathologists blinded to patients’ clinical features under a fluorescence microscope (BX43; Olympus, Japan) equipped with a DAPI/green/orange triple band pass filter and a microscope digital camera (DP50; Olympus, Japan). IKZF3, HER2 and CEP17 signals were counted in at least 100 tumor cell nuclei of each GC sample under an oil microscope at 1,000x magnification. Amplification of IKZF3 was defined according to previously documented scoring algorithms for HER2 (Balestra et al. 2023). That is, IKZF3/CEP17 ratio ≥ 2.0.
Immunohistochemistry (IHC)
The IHC assay using IKZF3 rabbit monoclonal antibody (Abcam, ab139408) was administered on the TMA sections with the Ventana iView DAB Detection Kit on a BenchMark XT automated staining system (Ventana Medical Systems, Tucson, AZ). Any nuclear expression of IKZF3 is considered positive.
Statistical analysis
The statistical analysis were administered with GraphPad Prism 9.4.1. All P-values were two sided, a P ≤ 0.05 were considered statistically significant. Pearson χ2 test was performed to calculate the correlation between IKZF3 amplification and various clinicopathologic variables in GC patients. The Kaplan–Meier method with log-rank test was utilized to calculate the correlation between IKZF3/HER2 amplification and the survival ratio of OS and DFS, and to determine whether the survival curves were significantly different. The Cox proportional hazard regression model was administered for both univariate and multivariate analysis, with the hazard ratio (HR) were obtained.
Results
Clinical and pathological features
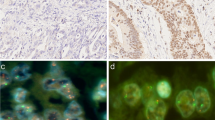
We summarized the main clinical and pathological features of the 404 patients with GC (Table 1). The mean age was 63.5 years (overall range 24 to 84 years). Among these GC patients, 298 (73.8%) were male. pTNM stage was determined based on pathologic evaluations, 222 cases (54.9%) were staged I & II and 182 (45.1%) cases were staged III & IV. Vessel invasions were identified in 200 (49.5%) cases, neural invasions in 189 (46.8%) cases. Lymph node metastasis was observed in 61.1% (247 of 404) of GC patients. For histologic subtyping in GC, a further classification was made according to Lauren’s criteria. 196 (48.5%) cases of intestinal-type (Fig. 1A), 179 (44.3%) cases of mixed-type (Fig. 1B), and 22 (5.44%) cases of diffuse-type were identified. During the follow-up period, 37.3% (151 of 404) patients developed disease progression. 23.8% (96 of 404) patients died of GC.
(A-B) Representative images of H&E-staining (×200) in GC patients. A Intestinal-type GC, B Mix-type GC. (C-F) Representative patterns of IKZF3 or HER2 gene (orange color) and CEP17 (green color) status by FISH (×1,000). CIKZF3 non-amplification, D IKZF3 amplification. E HER2 non-amplification, FHER2 amplification. (G-H) Kaplan–Meier curves of poorer disease-free survival (DFS) and overall survival (OS) of cases with IKZF3 amplification in 404 GC patients. (I-J) Kaplan–Meier curves indicate no difference in DFS and OS between cases with or without HER2 amplification
The relevance between IKZF3 amplification and clinicopathological characteristics of GC patients
We evaluated IKZF3 amplification in 404 patients with GC by FISH. IKZF3 amplification (IKZF3/CEP17 ratio ≥ 2.0) was found in 6.9% (28 of 404) of patients (Fig. 1D), and other patients (93.1%, 376 of 404) showed non-amplification (Fig. 1C). The associations between IKZF3 amplification and related clinicopathological characteristics are presented in Table 1. IKZF3 amplification status was found significantly correlated with intestinal-type GC (P = 0.001), disease progression (P = 0.03), and HER2 amplification (P = 0.0001) (Fig. 1E-F). There was no significant difference between IKZF3 amplification and IKZF3 wild type group considering sex (P = 0.14), age (P = 0.35), pTNM stage (P = 0.81), differentiation grading (P = 0.66), lymph node metastasis (P = 0.45), EBV infection (P = 0.62), tumor deposits (P = 0.89), vessel invasion (P = 0.74), nerve invasion (P = 0.41), and cancer related death (P = 0.12). Given the strong correlation between IKZF3 amplification and intestinal-type GC, we then analyzed its association with lymph node metastasis and vessel invasion in early-stage GC patients (Tis + T1a + T1b). IKZF3 amplification was found not related to LNM, but significantly correlated with vessel invasion in early-stage GC patients (Table 1). Additionally, IHC assay was performed on the TMA sections to assess IKZF3 protein expression, with the results that low protein expression of IKZF3 was detected in 12 cases with IKZF3 amplification, while no IKZF3 expression was detected in the remaining 16 cases with IKZF3 amplification (Assessed by two senior pathologists) (Supplemental information Fig. 1). This proves the protein expression of IKZF3 does not significantly correlate with amplification status.
IKZF3 amplification is associated with poor prognosis in GC patients
Survival data were analyzed of all 404 GC patients. The 5-year DFS and OS rates were 56.7% (229/404) and 58.9% (238/404) respectively, the median follow-up period is 63.66 months (range 1–82.5 months). Mean and median duration of DFS were 48.9 and 62.9 months, while to OS were 52.1 and 64.2 months respectively. To further investigate the prognostic value and clinical outcomes of IKZF3 amplification in GC, Kaplan–Meier analysis was performed with the results that compared with the group without IKZF3 amplification (n = 376, median DFS, 50.9 months; median OS, 53.8 months), cases with IKZF3 amplification (n = 28) gained significant worse survival with a median DFS 39.7 months (P = 0.014) and median OS 45.7 months (P = 0.018) respectively (Fig. 1G-H). As a comparison, the survival data of all GC patients with HER2 amplification were also analyzed, and the results showed no significant difference between GC patients with or without HER2 amplification in terms of both DFS (P = 0.47) and OS (P = 0.39) (Fig. 1I-J). Besides, univariate analysis of prognostic significance suggests that age, pTNM stage, Lauren classification, LN metastasis, IKZF3 amplification, tumor deposits, vessel invasion and nerve invasion were significantly associated with DFS and OS in all GC patients (Table 2). In the multivariate analysis, age (P = 0.002, HR: 0.485 for DFS; P = 0.001, HR: 0.466 for OS), pTNM stage (P = 0.032, HR: 1.806 for DFS; P = 0.023, HR: 1.868 for OS), LN metastasis (P = 0.029, HR: 2.246 for DFS; P = 0.035, HR: 2.184 for OS), IKZF3 amplification (P = 0.006, HR: 2.286 for DFS; P = 0.004, HR: 2.416 for OS), tumor deposits (P < 0.001, HR: 1.322 for DFS; P < 0.001, HR: 1.300 for OS), vessel invasion (P = 0.04, HR: 1.611 for DFS; P = 0.03, HR: 1.655 for OS), and nerve invasion (P = 0.021, HR: 1.678 for DFS; P = 0.012, HR: 1.750 for OS) were associated with DFS and OS (Table 2). These findings imply that IKZF3 amplification stands as an independent prognostic factor across all 404 GC cases.
In our study, twenty-two out of 28 patients with IKZF3 amplification were histologically classified as IGC referring to Lauren classification. Therefore, we administered relevant survival analysis among GC patients from different Lauren subtypes. In IGC patients (n = 196, Fig. 2A-B), IKZF3 amplification was discovered to exhibit a substantial correlation with unfavorable outcomes, with a median DFS and OS being 37.1 and 44.0 months compared with 52.8 and 54.9 months for 174 patients without IKZF3 amplification (P < 0.001 for DFS and P < 0.001 for OS). In other types GC patients (n = 208, Fig. 2C-D), IKZF3 amplification did not have a significant impact on DFS (P = 0.44) or OS (P = 0.43). The median duration of DFS and OS for 6 patients with IKZF3 amplification was 42.3 and 48.5 months respectively, compared to 49.6 and 53.0 months for the remaining 202 patients without IKZF3 amplification. It is worth pointing out that in IGC, patients with HER2 amplification (n = 41) have poorer DFS (P = 0.008) and OS (P = 0.01) compared to those without HER2 amplification (n = 155, Fig. 2E-F). However, in other types of GC patients, HER2 amplification is not significantly correlated with patient prognosis (P = 0.26 for DFS, P = 0.39 for OS) (n = 208, Fig. 2G-H). Univariate analysis suggests that pTNM stage, HER2 amplification, LN metastasis, IKZF3 amplification, tumor deposits, vessel invasion, and nerve invasion were significantly linked to DFS and OS in IGC patients (Table 2). In the multivariate analysis, IKZF3 amplification (P = 0.007, HR: 3.381 for DFS; P = 0.004, HR: 3.734 for OS) and tumor deposits (P < 0.001, HR: 1.334 for DFS; P = 0.003, HR: 2.519 for OS) were associated with DFS and OS (Table 2). These data demonstrate that IKZF3 amplification holds an independent prognostic value for survival in IGC patients. Meanwhile HER2 amplification is linked to unfavorable prognosis in IGC patients, whereas not an independent prognostic factor.
Survival analyses based on Lauren classification of GC patients. (A, B) IKZF3 amplification was associated to poorer DFS and OS in IGC patients. (C, D) IKZF3 amplification was not corelated with DFS and OS in other types GC patients. (E, F) HER2 amplification was linked to poorer DFS and OS in IGC patients. (G, H) HER2 amplification was not associated to DFS and OS in other types GC patients
Furthermore, in stages I–II IGC patients (n = 126, Fig. 3A-B), IKZF3 amplification was correlated with poor DFS (P < 0.001) and OS (P < 0.001) significantly. A worse prognosis was observed in 12 patients with IKZF3 amplification, with a median DFS and OS being 43.8 and 52.8 months compared with 57.6 and 58.6 months for 114 patients without IKZF3 amplification. In stages III–IV IGC patients (n = 65, Fig. 3C-D), IKZF3 amplification also showed poor clinical outcomes, with the median DFS and OS were 29.1 and 33.4 months respectively in 10 patients with IKZF3 amplification, whereas it was 44.1 and 48.4 months respectively for 55 patients without IKZF3 amplification (P = 0.02 for DFS and P = 0.01 for OS). On the other hand, HER2 amplification exhibit significantly worse DFS and OS in stages I–II (n = 126, Fig. 3E-F) IGC patients, however not in stages III–IV (n = 65, Fig. 3G-H). Besides, in stages I–II IGC patients, univariate analysis suggests that HER2 amplification, LN metastasis, IKZF3 amplification, tumor deposits, and nerve invasion were significantly associated with DFS and OS (Supplemental information (SI) Table 1). In the multivariate analysis, IKZF3 amplification (P = 0.019, HR: 3.737 for DFS; P = 0.036, HR: 3.203 for OS) and LN metastasis (P = 0.013, HR: 3.728 for DFS; P = 0.018, HR: 3.345 for OS) were associated with DFS and OS (SI Table 1). In stages III-IV IGC patients, univariate analysis suggests that age, IKZF3 amplification, and tumor deposits exhibit correlation with both DFS and OS (SI Table 1). In the multivariate analysis, IKZF3 amplification (P = 0.045, HR: 2.320 for DFS; P = 0.865, HR: 0.881 for OS) and tumor deposits (P = 0.007, HR: 1.286 for DFS; P = 0.26, HR: 1.25 for OS) were associated with DFS, but not with OS (SI Table 1). These data demonstrate that IKZF3 amplification has independent prognostic significance for survival outcomes in IGC patients, regardless of patients in stages I-II or III-IV. HER2 amplification is linked to poor prognosis in stages I-II IGC patients, but it was not identified as an independent prognostic factor (Table 2 and SI Table 1).
Ultimately, we conducted a comprehensive analysis of the prognosis in GC patients with co-amplification of IKZF3 and HER2, and significant reductions were observed in both DFS and OS among all 404 GC patients (Fig. 4A-B) as well as IGC patients (Fig. 4C-D). Moreover, within the subset of patients with HER2 amplification, those also exhibiting IKZF3 amplification displayed a notably poorer prognosis (Fig. 4E-F). These findings suggest the additional clinical significance of IKZF3 amplification in stratifying HER2 amplified GC patients. However, it is worth noting that among patients with IKZF3 amplification, there was no discernible impact on patient prognosis based on their HER2 gene status (Fig. 4G-H).
(A-D)IKZF3 and HER2 co-amplification was associated with poorer DFS and OS for 404 patients (A-B) and 196 IGC patients (C-D). (E-F) IKZF3 amplification in HER2-amplified GC patients was related to potential poorer DFS and OS. (G-H) HER2 amplification in IKZF3-amplified GC patients was not related to poorer DFS and OS
Discussion
Clinical treatment and prognosis of individuals diagnosed with GC are influenced by various factors, including pathological diagnosis, clinical staging, molecular pathological classification, etc. In most cases, patients with the same histologic type might display distinct molecular phenotypes and prognosis. It’s generally known that many non-anatomic factors, such as genetic and protein markers related to carcinogenesis and tumor invasion, have also been confirmed to have significant clinical implications for prognosis (Patel 2020). Therefore, accurately identifying new biomarkers in GC from multiple perspectives holds great prognostic significance and is more conducive to personalized therapy for GC patients after surgical treatment.
Many common amplifications in gastric cancer remain poorly elucidated, which could be crucial for us to understand the occurrence, development, invasion, as well as diagnosis and prognosis of GC. In this study, we attempted to explore common gene amplification events which might be of clinical significance for GC patients. We queried stomach cancer databases (TCGA, Nature 2014) deposited in cBioPortal, and the frequency of all amplified genes was ranked. An arbitrary cut-off of 10% frequency was used for gene amplification, IKZF3 was selected for further analysis in 14 genes. IKZF3 is a hematopoietic specific transcription factor that plays a pivotal role in the differentiation, proliferation, and development of both B and T lymphocytes (Li et al. 2018). In the field of solid tumours, bioinformatics-based research has demonstrated that IKZF3 serves as an independent prognostic factor for both skin melanoma (SKCM) and human head and neck squamous cell carcinoma (HNSCC) (Li et al. 2023; Yang et al. 2022), suggesting its potential as a promising target for immunotherapy in the treatment of SKCM and HNSCC. However, the research on the association between IKZF3 amplification and GC is scarce. In our study, IKZF3 was found to amplified in 6.9% of GC patients, and it served as an independent prognostic marker indicating unfavorable outcomes, especially in IGC. The prognostic value of IKZF3 was superior to HER2 for GC patients.
In our study, patients with or without IKZF3 amplification account for 6.9% (n = 28) and 93.1% (n = 376) respectively of all the 404 GC patients. We were surprised that IKZF3 amplification showed a significant positive correlation with intestinal type of Lauren classification and HER2 amplification (Table 1). Moreover, IKZF3 amplification is significantly associated with vessel invasion in early gastric cancer, this indicate IKZF3 amplification could potentially be a clue to distinguish between endoscopic submucosal dissection (ESD) and gastrectomy cases. Notably, IGC accounts for 78.6% (22/28) of GC patients with IKZF3 amplification, and 63.1% (41/65) of GC patients with HER2 amplification. In previous studies, HER2 expression had been observed to be higher in IGC compared to other types (Cruz-Reyes et al. 2013; Joshi et al. 2021), which is consistent to our results. On the other hand, HER2 amplification accounts for 75.0% (21/28) in GC with IKZF3 amplification. And conversely, IKZF3 non-amplification group were about 88% HER2 negative. This result aligns with the findings of preceding investigations (Lin et al. 2022), a bioinformatics-based study which involved these two genes in breast cancer, at a co-amplification rate of about 12–15%. This might be the result of that IKZF3 is located in close proximity to the proposed ERBB2 17q12-q21 amplicon. This implies that IKZF3 amplification in GC has certain differential diagnostic value.
As is well known, intestinal type (tubular and papillary) adenocarcinoma has a more favorable prognosis (Chen et al. 2015). Commonly associated with atrophic gastritis and gastric epithelial dysplasia, may express HER2 or PD-L1, both of which offer additional treatment options. Other types of GC, for instance diffuse type gastric carcinoma, consists of signet ring cells that are poorly differentiated, present as scattered individual cells or clusters, which is related to aggressive tumor proliferation, peritoneal metastasis, chemo resistance, and poor prognosis. In IGC instead of in other types of GC, IKZF3 amplification is related to dismal prognosis, and act as an independent prognostic indicator in IGC, irrespective of the pTNM stages being I-II or III-IV (Fig. 3A-D). On the other hand, it is interesting to note that compared to other types of GC, HER2 amplification is significantly linked to an unfavorable outcome in IGC, exclusively in stages I-II, but not in full cohort (Figs. 1I-J and 3E-H). In light of the evidence, it can be reasonably inferred that IKZF3 amplification even outperformed HER2 in predicting patient prognosis to some extent. Besides, among patients with HER2 amplification, those who also exhibit IKZF3 amplification have a worse prognosis (Fig. 4E-F), highlighting the clinical significance of IKZF3 amplification in stratifying HER2-amplified GC patients.
Bioinformatics-based analysis shows HER2 amplification, along with several co-amplified genes within a focal region, also known as the HER2 amplicon, was determined to be a six-gene area including PGAP3-ERBB2(HER2)-MIR4728-MIEN1-GRB7-IKZF3 (Li et al. 2020). In our current investigation, HER2 amplification was observed in approximately 75% of cases with IKZF3 amplification, which suggests the intriguing possibility that in addition to synergistically amplified with HER2, IKZF3 amplification can also operate as an independent amplicon.
Generally, HER2 is amplified in approximately 10–20% of gastric cancers (Boku 2014). Although 30 years have passed since the initial studies revealing a correlation between a positive HER2 status and unfavorable prognosis were published, there remains ongoing debate surrounding this matter (Heike Grabsch 2010; Kurokawa et al. 2014; Lei et al. 2017; Wang et al. 2017). This may due to inadequate sample size, variations in population demographics, tumor heterogeneity, and discrepancies in assessment methods utilized across various studies, including antibody clones, evaluation criteria, and cut-offs applied for defining HER2 positivity. Nevertheless, GC patients with HER2 amplification gained improved overall survival on first-line trastuzumab–based therapy in recent years (Boku 2014; Joshi et al. 2021). However, approximately 30–60% patients experiencing disease progression after treatment with trastuzumab-based therapies exhibit HER2 downregulation (Kanayama et al. 2018; Kim 2024; Sampera et al. 2019), it may result from either high percentage of primary and acquired resistance, which involves deregulation of PI3K/Akt pathway, HER2 loss and heterogeneity of HER2 proteome, or co-amplifications of RTK/KRAS, MET, CCNE1 (Joshi et al. 2021; Röcken 2022; Zhu et al. 2021). Our current research findings indicate simultaneous assessment of HER2 and IKZF3 gene status is indispensable for targeted therapy in GC, this can further stratify the risk of GC patients, evaluate patient prognosis and guide subsequent medication plans. Moreover, this may holds promise in improving clinical outcomes for patients with IKZF3/HER2 co-amplification, which constitutes the main focus of our study. Based on our current results, screening drugs that target IKZF3 amplification or IKZF3/HER2 co-amplification, which can be developed and introduced into clinical practice is of important significance to GC patients, this part is currently in progress. This is also our main research direction in the future.
Nevertheless, our research has certain limitations. First, the use of TMA technique can underestimate or overestimate IKZF3 status due to tumor and microenvironment heterogeneity. Second, in this study, the amplification cut-off for IKZF3 was aligned with that of HER2, which was determined based on the efficacy of the targeted drug. Further research is needed to establish the optimal IKZF3 amplification cut-off value for prognostic prediction. Third, IKZF3 protein level could not reflect IKZF3 amplification status in our research. This is a very interesting point, the potential mechanisms including single nucleotide polymorphism in regulatory regions, post-transcriptional and post-translational modifications, mutations affecting upstream signaling pathways, as well as mutations that regulate protein stability. Further experiments are required to clarify this inconsistent mechanism.
This article is the first to elucidate the value of IKZF3 amplification as a novel prognostic factor in GC patients, especially in IGC. Accordingly, we believe it is essential to trace the upstream and downstream signaling pathways associated with IKZF3 to gain a comprehensive understanding of its role in the occurrence and development of GC as well as its implications in patient prognosis. In vitro and in vivo laboratory experiments are warranted in future.
Conclusion
To summarize, our study revealed that IKZF3 amplification is an independent prognostic factor in GC, which is superior to HER2 to some extent. The prognostic significance of IKZF3 requires more test-proof evidences in the future, which offers perspective on a potential role of IKZF3 as a promising druggable target for GC as well as other carcinomas.
Data availability
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
References
Albertson DG (2006) Gene amplification in cancer. Trends Genet 22(8):447–455
Aravind Sanjeevaiah NC, Hester C, Matthew R, Porembka (2018) Gastric Cancer: recent molecular classification advances, racial disparity, and Management implications. Clin Rev 14(4):217–224
Awwad MHS, Kriegsmann K, Plaumann J, Benn M, Hillengass J, Raab MS et al (2018) The prognostic and predictive value of IKZF1 and IKZF3 expression in T-cells in patients with multiple myeloma. OncoImmunology 7(10):1–11
Balestra A, Larsimont D, Noël J-C (2023) HER2 amplification in p53-Mutated endometrial carcinomas. Cancers 15(5):1–6
Boku N (2014) HER2-positive gastric cancer. Gastric Cancer 17(1):1–12
Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS et al (2015) Clinicopathological variation of Lauren classification in gastric Cancer. Pathol Oncol Res 22(1):197–202
Chia NY (2016) P T Molecular Classification of Gastric Cancer. Annals of Oncology Advance Access 4(1–26
Cruz-Reyes C, Gamboa-Dominguez A (2013) Her2 amplification in gastric Cancer is a rare event restricted to the intestinal phenotype. Int J Surg Pathol 21(3):240–246
Douglas HRW (2000) The Hallmarks of Cancer. Cell 100(2):57–70
Heike Grabsch SS, H G a W M (2010) HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value – conclusions from 924 cases of two independent series. Cell Oncol 32(5):57–65
Heizmann B, Kastner P, Chan S (2018) The Ikaros family in lymphocyte development. Curr Opin Immunol 51(4):14–23
Hou G, Song B (2019) Gastric cancer patient with c-MET amplification treated with crizotinib after failed multi-line treatment: a case report and literature review. Math Biosci Eng 16(5):5923–5930
Joshi SS, Badgwell BD (2021) Current treatment and recent progress in gastric cancer. CA Cancer J Clin 71(3):264–279
Kanayama K, Imai H, Usugi E, Shiraishi T, Hirokawa YS, Watanabe M (2018) Association of HER2 gene amplification and tumor progression in early gastric cancer. Virchows Arch 473(5):559–565
Kim I (2024) Emerging targets for systemic treatment of gastric Cancer: HER2 and Beyond. J Gastric Cancer 24(1):29–56
Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A et al (2008) FGFR2-Amplified gastric Cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res 68(7):2340–2348
Kurokawa Y, Matsuura N, Kimura Y, Adachi S, Fujita J, Imamura H et al (2014) Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer 18(4):691–697
Lauren P (1965) The two histological main types of gastric carcinoma:diffuse and so-called intestinal-type carcinoma. Acta path et microbiol Scandinav 64(2):31–49
Lazarian G, Yin S, Ten Hacken E, Sewastianik T, Uduman M, Font-Tello A et al (2021) A hotspot mutation in transcription factor IKZF3 drives B cell neoplasia via transcriptional dysregulation. Cancer Cell 39(3):380–393
Lei Y, Huang J, Zhao Q, Jiang N, Xu H, Wang Z et al (2017) The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: a meta-analysis of literature. World J Surg Oncol 15(1):1–7
Li L, Ding X, Wang X, Yao Q, Shao X, An X et al (2018) Polymorphisms of IKZF3 gene and autoimmune thyroid diseases: Associated with Graves’ Disease but not with Hashimoto’s Thyroiditis. Cell Physiol Biochem 45(5):1787–1796
Li Z, Chen S, Feng W, Luo Y, Lai H, Li Q et al (2020) A pan-cancer analysis of HER2 index revealed transcriptional pattern for precise selection of HER2-targeted therapy. eBioMedicine 62(8):1–15
Li H, Ye M, Hu Z, Lu H, Zheng D, Wu M et al (2023) IKZF3 is a novel prognostic biomarker for head and neck squamous cell carcinoma: a study based on bioinformatics analysis. Med (Baltim) 102(11):1–12
Lin CY, Yu CJ, Shen CI, Liu CY, Chao TC, Huang CC et al (2022) IKZF3 amplification frequently occurs in HER2-positive breast cancer and is a potential therapeutic target. Med Oncol 39(12):242
Liu Y, Chen X, Chen X, Yang X, Song Q, Wu H (2019) High SALM3 expression in Tumor cells and fibroblasts is correlated with poor prognosis in gastric Cancer patients. Dis Markers 2019(4):1–8
Meng C, Chen S, He Q, Tan J, Wu J, Zhao J (2023) IKZF3 modulates cerebral ischemia/reperfusion injury by inhibiting neuroinflammation. Int Immunopharmacol 114(7):1–12
Nakata S, Fujita M, Nakanishi H (2019) Efficacy of Afatinib and Lapatinib Against HER2 gene-amplified trastuzumab-sensitive and -resistant human gastric Cancer cells. Anticancer Res 39(11):5927–5932
Oshima Y, Tanaka H, Murakami H, Ito Y, Furuya T, Kondo E et al (2014) Lapatinib sensitivities of two novel trastuzumab-resistant HER2 gene-amplified gastric cancer cell lines. Gastric Cancer 17(3):450–462
Patel TH, C M (2020) Targeted therapies in Advanced Gastric Cancer. Curr Treat Options Oncol 21(9):1–14
Röcken C (2022) Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol 149(1):467–481
Sampera A, Sánchez-Martín FJ, Arpí O, Visa L, Iglesias M, Menéndez S et al (2019) HER-Family ligands promote Acquired Resistance to Trastuzumab in Gastric Cancer. Mol Cancer Ther 18(11):2135–2145
Shinmura K, Kiyose S, Nagura K, Igarashi H, Inoue Y, Nakamura S et al (2014) TNK2 gene amplification is a novel predictor of a poor prognosis in patients with gastric cancer. J Surg Oncol 109(3):189–197
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F (2020) Gastric cancer. Lancet 396(10251):635–648
Song Z, Wu Y, Yang J, Yang D, Fang X (2017) Progress in the treatment of advanced gastric cancer. Tumour Biol 39(7):1–7
Ughetto S, Migliore C, Pietrantonio F, Apicella M, Petrelli A, D’Errico L et al (2021) Personalized therapeutic strategies in HER2-driven gastric cancer. Gastric Cancer 24(4):897–912
Wang H-B, Liao X-F, Zhang J (2017) Clinicopathological factors associated with HER2-positive gastric cancer. Medicine 96(44)
Yang LK, Lin CX, Li SH, Liang JJ, Xiao LL, Xie GH et al (2022) Novel IKZF3 transcriptomic signature correlates with positive outcomes of skin cutaneous melanoma: a pan-cancer analysis. Front Genet 13(6):1–16
Yuan Shi DH, Yingyong H (2013) An alternative high output tissue microarray technique. Diagn Pathol 8(9):1–6
Zhu Y, Zhu X, Wei X, Tang C, Zhang W (2021) HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer 1876(1):1–14
Funding
This work was supported by National Key R&D Programs of China (2021YFF1201001), the National Key Clinical Specialty Discipline Construction Program of China (No. YWP2023-001) and Shanghai Municipal Key Clinical Specialty Foundation (No. shslczdzk01302).
Author information
Authors and Affiliations
Contributions
Y.H. and C.X. initiated the project, Z.C. designed and administered the study, R.L constructed TMAs, R.L, W.Y, W.H, L.Z. and H.L provide clinical information, pathologic assessment as well as follow-up data, H.L did literature survey, Z.C, L. L, J. S, J. H. performed FISH assays and analyzed all data. The draft was written by Z.C, H.L revised the manuscript. All authors reviewed and made revisions to the final manuscript. All authors contributed to the article and provided their approval for its submission.
Corresponding authors
Ethics declarations
Ethical approval
All studies involving human participants were reviewed and approved by the Human Research Ethics Committee of Zhongshan Hospital, Fudan University.
Informed consent
Written informed consent was obtained from patients for use of their surgical specimens for research purposes.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cui, Z., Liang, H., Luo, R. et al. IKZF3 amplification predicts worse prognosis especially in intestinal-type gastric cancer. J Cancer Res Clin Oncol 150, 363 (2024). https://doi.org/10.1007/s00432-024-05868-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05868-2