Abstract
Purpose
The SARS-CoV-2 Omicron variant of concern (VOC) and subvariants like BQ.1.1 demonstrate immune evasive potential. Little is known about the efficacy of booster vaccinations regarding this VOC and subvariants in cancer patients. This study is among the first to provide data on neutralizing antibodies (nAb) against BQ.1.1.
Methods
Cancer patients at our center were prospectively enrolled between 01/2021 and 02/2022. Medical data and blood samples were collected at enrollment and before and after every SARS-CoV-2 vaccination, at 3 and 6 months.
Results
We analyzed 408 samples from 148 patients (41% female), mainly with solid tumors (85%) on active therapy (92%; 80% chemotherapy). SARS-CoV-2 IgG and nAb titers decreased over time, however, significantly increased following third vaccination (p < 0.0001). NAb (ND50) against Omicron BA.1 was minimal prior and increased significantly after the third vaccination (p < 0.0001). ND50 titers against BQ.1.1 after the third vaccination were significantly lower than against BA.1 and BA.4/5 (p < 0.0001) and undetectable in half of the patients (48%). Factors associated with impaired immune response were hematologic malignancies, B cell depleting therapy and higher age. Choice of vaccine, sex and treatment with chemo-/immunotherapy did not influence antibody response. Patients with breakthrough infections had significantly lower nAb titers after both 6 months (p < 0.001) and the third vaccination (p = 0.018).
Conclusion
We present the first data on nAb against BQ.1.1 following the third vaccination in cancer patients. Our results highlight the threat that new emerging SARS-CoV-2 variants pose to cancer patients and support efforts to apply repeated vaccines. Since a considerable number of patients did not display an adequate immune response, continuing to exhibit caution remains reasonable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies report an increased risk for severe coronavirus-19 disease (COVID-19) in patients with cancer and in those with weakened immune systems as compared to individuals without cancer (Lee et al. 2020; Williamson et al. 2020; Rüthrich et al. 2021). With the implementation of global vaccination strategies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), vaccinating cancer patients has been shown to decrease the risk of hospitalization and mortality due to COVID-19. Moreover, early results on seroconversion following SARS-CoV-2 vaccination in cancer patients demonstrated promising results (Addeo et al. 2021; Shroff et al. 2021). However, immunogenicity was reduced compared to healthy subjects and was especially low in patients with hematological diseases or those treated with B cell depleting therapy (i.e., anti-CD20 therapy) (Giuliano et al. 2022; Nooka et al. 2022). Furthermore, titers of neutralizing antibodies (nAb) against variants of concern (VOC) were reduced compared to wild-type SARS-CoV-2 (Fendler et al. 2021; Terada et al. 2022) and levels of both IgG anti-spike (anti-S) antibodies and nAb against VOC reportedly decrease six months post vaccination (Obeid et al. 2022). Consequently, additional booster vaccinations are recommended to address this deficit (Bar-On et al. 2022; Davis-Gardner et al. 2022; Koch et al. 2022). While it has been shown that a third vaccination increases levels of IgG anti-S antibodies as well as nAb in both healthy subjects and cancer patients (Pajon et al. 2022), immune response remains low or undetectable in some patients, especially in those with B cell malignancies or anti-CD20 therapy (Lim et al. 2022). Of interest, the Omicron VOC exhibits extensive immune evasive potential (Cao et al. 2022; Cele et al. 2022; Hoffmann et al. 2022) and low or undetectable levels of nAb against this VOC were reported despite two doses of mRNA vaccines (Chang et al. 2022; Edara et al. 2022; Garcia-Beltran et al. 2022). Furthermore, Omicron subvariants like BQ.1/BQ.1.1, which has become one of the predominant VOC during winter of 2022/2023, are emerging and have demonstrated an even increased resistance to both nAb and therapeutic monoclonal antibodies (Arora et al. 2022a, b; Kurhade et al. 2022; Planas et al. 2022; Qu et al. 2022; Wang et al. 2022). However, little is known about the efficacy of a booster vaccination against the Omicron VOC in cancer patients and so far, data concerning neutralization capacity against subvariant BQ.1.1 following booster vaccination in immunocompromised patients are limited (Ehmsen et al. 2023). The present study, therefore, aims to add to the body of evidence of the impact of a third vaccination on anti-SARS-CoV-2 antibodies and their neutralizing capacity on Omicron VOC (BA.1, BA.4/5 and BQ.1.1) in a representative cohort of cancer patients.
Methods
Between 01/2021 and 02/2022, unselected cancer patients treated at our Ruhr University oncology center were prospectively included in our local COVID-19 biobanking study. All patients agreed to provide baseline medical data and multiple blood samples. Additionally, a subset of patients opted to provide further information via questionnaires (Overheu et al. 2022). As depicted in Fig. 1, sera were collected at the following time points: t(1): prior to the first vaccination, t(2): after the first vaccination, t(3): after the second vaccination, t(4): after 3 months, t(5): after 6 months, t(6): prior to third vaccination, t(7): after third vaccination. Blood serum samples were stored on-site at − 80 °C.
Timeline of data collection with mean time since or to vaccination and number of samples collected at each time point; t(1): prior to 1st anti-SARS-CoV-2 vaccination, t(2): after 1st vaccination, t(3): after 2nd vaccination, t(4): follow-up at 3 months, t(5): follow-up at 6 months, t(6): prior to third vaccination, t(7): after third vaccination; S: solid tumors, H: hematologic malignancies
Laboratory analyses were performed at the Department of Molecular and Medical Virology, Ruhr University Bochum and the Institute of Clinical Hygiene, Medical Microbiology and Infectiology at Klinikum Nürnberg, Paracelsus Medical University.
The study was approved by the Ethics Committee of the Medical Faculty, Ruhr University Bochum (reference number 20-6953-bio and 21-7351) and conducted in accordance with the Declaration of Helsinki. Descriptive data are presented as n (%) or median (range or standard deviation). All own percentual results are rounded to the nearest full number. Data were analyzed using Fisher’s exact test, Student’s and Welch’s t test (depending on data variance) or χ2 test. Correlations were evaluated using Pearson’s correlation coefficient test and multivariate linear or logistic regression. Results were considered significant at α = 0.05. All analyses were performed using SPSS (v. 26) and GraphPad PRISM for Windows (v. 9.5.0).
SARS-CoV-2 IgG anti-S antibodies
For the detection of SARS-CoV-2 spike-1-specific IgG concentrations, the Euroimmun enzyme-linked immunosorbent assay (ELISA) Anti-SARS-CoV-2-QuantiVac-ELISA was used according to the manufacturer’s instructions (Euroimmun AG, Lübeck, Germany). Positive and negative controls were included in each test run. Quantification of S1‐specific IgG was performed using a 6‐point calibration curve covering a range from 1 to 120 relative units (RU)/ml. By multiplication with factor 3.2, results in RU/ml were converted into standardized binding antibody units (BAU)/ml. Results < 25.6 were considered negative, ≥ 25.6–< 35.2 borderline, and ≥ 35.2 positive.
SARS-CoV-2 neutralizing antibody ELISA
To determine nAb against parental (wildtype) SARS-CoV-2-Spike in patient sera, a competitive ELISA Kit from Invitrogen (#BMS2326) was used. The ELISA was performed and evaluated according to manufacturer instructions. Briefly, receptor binding domain (RBD) pre-coated ELISA plates were incubated with patient sera to specifically bind nAb. Afterwards, the plates were incubated with biotinylated ACE2. Streptavidin-HRP conjugate was used with a chromogene substrate to detect ACE2-RBD binding with a plate reader (Berthold). ELISA results were used to select the patient cohort for the following determination of neutralizing antibody titers (PVND50) for Anti-SARS-CoV2-S antibodies against Omicron subvariants BA.1 (B 1.1.529), BA.4/5 and BQ1.1 (sera included with neutralizing capacities > 20% in the ELISA).
SARS-CoV-2 neutralization pseudovirus assay
Expression plasmids harboring the pCG1-SARS-CoV- 2 BA.1 (B 1.1.529) SΔ18 (codon-optimized, C-terminal truncation of 18 amino acid residues, GISAID Accession ID: EPI_ISL_6640919), pCG1-SARS-CoV-2 BA.4/5 SΔ18 (codon-optimized, C-terminal truncation of 18 amino acid residues, GISAID Accession ID: EPI_ISL_11550739 and EPI_ISL_12029894) and SARS-CoV-2 BQ.1.1 SΔ18 (GISAID Accession ID: EPI_ISL_14752457) were kindly provided by S. Pöhlmann and M. Hoffmann (German Primate Center-Leibniz Institute for Primate Research, Göttingen, Germany) and have been described before (Arora et al. 2022a, b; Hoffmann et al. 2022). VSV*∆G(FLuc)-pseudoparticles, harboring the BA.1, BA.4/5 or BQ.1.1 spike were produced as previously described (Zettl et al. 2020). Briefly, the rhabdoviral pseudotyped particles were produced in 293 T cells transfected to express the Omicron SARS-CoV-2-S subvariants and inoculated with VSV*∆G-FLuc, a replication-deficient vesicular stomatitis virus (VSV) vector that encodes for enhanced green fluorescent protein and firefly luciferase (FLuc) instead of VSV-G protein (kindly provided by Gert Zimmer, Institute of Virology and Immunology, Mittelhaeusern, Switzerland). Pseudoparticles were collected, cleared from cellular debris by centrifugation and stored at − 80 °C until further use. For the virus neutralization assay, sera were incubated for 30 min at 56 °C to inactivate complement factors. SARS-CoV-2 pseudoparticles were incubated with triplicates of twofold serial dilutions of immune sera in 96-well plate prior to infections of Vero E6 cells (1 × 104 cells/well) in DMEM + 10% FBS (Life Technologies). At 18 h post infection, firefly luciferase (FLuc) reporter activity was determined and the reciprocal antibody dilution causing 50% inhibition of the luciferase reporter calculated (PVND50; lower limit of detection: 20 PVND50; upper limit of detection: 2560 PVND50). Analysis for nAb against subvariants BA.4/5 and BQ.1.1 was only performed for sera collected after the third vaccination.
Results
Patient characteristics
A total of 148 patients (41% female) were included. Baseline characteristics are presented in Table 1. Mean age was 63.9 (24–87) years. Most patients suffered from solid tumors (n = 126, 85%), mainly gastrointestinal (n = 87, 59%). The most frequent diagnosis was pancreatic cancer (n = 59, 40%). Most patients were on active cancer therapy (n = 136, 92%), mainly chemotherapy (n = 118, 80%), only four (3%) patients received B cell-depleting therapy. History of COVID-19 was present in seven (5%) patients and five (3%) patients were diagnosed with COVID-19 during the study (one patient after basic immunization and four patients after third vaccination).
Most patients had BNT162b2 (85%) for their basic immunization. In total, 95 (64%) patients received a third SARS-CoV-2 vaccination (n = 76 (80%) BNT162b2, n = 19 (20%) mRNA-1273).
Humoral immune responses following vaccination
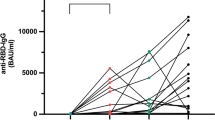
We analyzed a total of 408 serum samples (Fig. 1). Analysis of anti-SARS-CoV-2 IgG binding antibody units (BAU) demonstrated significantly increased antibody titers following the third vaccination compared with all other time points (p < 0.0001). Antibody levels were significantly lower following the first SARS-CoV-2 vaccination (p ≤ 0.009), except compared with the respective levels prior to the third vaccination (p = 0.076), indicating a decline in antibody titers over time (Fig. 2).
Following the third anti-SARS-CoV-2 vaccination, the proportion of neutralizing antibodies (nAb) against parental SARS-CoV-2 (wild-type) increased significantly (153.8 vs. 339.7 BAU/ml, p < 0.0001). Overall, 85% of patients elicited nAb with a neutralizing capacity > 20% after booster vaccination, compared to only 52% after 3 months (p < 0.001). The titers of nAb against wild-type SARS-CoV-2 after the third vaccination were significantly correlated with anti-SARS-CoV-2 IgG BAU titers (r = 0.813, p < 0.0001; Fig. 3A). ND50 titers against the Omicron subvariant BA.1 were significantly higher following third vaccination (p < 0.0001) and overall significantly correlated with anti-SARS-CoV-2 IgG antibody levels (r = 0.239, p < 0.0001; Fig. 3B) and proportion of nAb against parental (wild-type) SARS-CoV-2 (r = 0.3; p < 0.0001).
However, while ND50 titers after the third vaccination correlated significantly with their corresponding BAU titer levels after the third vaccination (r = 0.254, p = 0.048), they did not correlate with BAU levels at any other individual time point. ND50 titers against Omicron subvariants BA.4/5 and BQ.1.1 after third vaccination (n = 65) tended to correlate with corresponding BAU titers (BA.4/5: r = 0.22, p = 0.076; BQ.1.1: r = 0.187, p = 0.136) and significantly correlated with corresponding nAb titers against parental SARS-CoV-2 (BA.4/5: r = 0.29, p = 0.019; BQ.1.1: r = 0.268, p = 0.031). There were significant differences between mean ND50 titers against BA.1, BA.4/5 and BQ.1.1, with the latter being significantly lower compared to the former (36.78 vs. 241.3 vs. 621.3, p < 0.0001; Fig. 4). Nearly half of the patients evaluated for nAb against BQ.1.1 (31/65, 48%) and all evaluated patients with a hematologic malignancy did not demonstrate any detectable titer level.
Choice of booster SARS-CoV-2 vaccine did neither significantly influence nAb (BNT162b2 vs. mRNA-1273: 64% vs. 71%; p = 0.45) nor ND50 titers post-third vaccination (p = 0.43).
BAU titers (p = 0.008) and expression of nAb (32% vs. 58%; p = 0.002) were significantly higher among those with a history of COVID-19, while ND50 titers did not differ (p = 0.28). Patients with SARS-CoV-2 breakthrough infections had significantly lower BAU and nAb titers against parental SARS-COV-2, both at 6 months follow-up (12.1 vs. 179.6 BAU/ml, p < 0.001; 0% vs. 26%, p < 0.001) and after third vaccination (196.5 vs. 347.7 BAU/ml, p = 0.011; 29% vs. 69%, p = 0.018). The characteristics of the patients with breakthrough infections are displayed in Table 2.
Influence of cancer entity and therapy
Mean titers of BAU, nAb and ND50 were significantly lower in patients with hematologic diseases than in those with solid malignancies, both over all time points (197.9 vs. 103.3 BAU/ml; p < 0.0001; nAb: 17% vs. 35%, p < 0.0001; ND50: 38.4 vs. 275.1, p < 0.0001) and following third vaccination (196.6 vs. 356.4 BAU/ml, p = 0.028; nAb: 34% vs. 70%, p < 0.001; ND50: 50.9 vs. 672.2, p < 0.0001; Fig. 5). This also applied to ND50 titers against Omicron subvariant BA.4/5, but not BQ.1.1 after third vaccination (BA.4/5: 2.9 vs. 270.5, p < 0.001; BQ.1.1: 0 vs. 41.2, p < 0.001; Fig. 4).
Patients receiving B cell-depleting therapy had significantly lower BAU levels (63.4 vs. 190.6 BAU/ml; p < 0.0001). Accordingly, nAb titers were also significantly lower among patients receiving B cell-depleting therapy (9% vs. 33%; p < 0.0001). Treatment with chemotherapy (192.1 vs. 168.0 BAU/ml, p = 0.22) or immunotherapy (193.3 vs. 186.8 BAU/ml; p = 0.75) did not significantly influence antibody response over all time points, except for BAU and nAb against parental SARS-CoV-2 levels after the third vaccination, where titers were significantly higher among patients receiving chemotherapy (Fig. 6A/B).
Influence of various clinical factors
BAU and nAb titers were overall significantly negatively correlated with patients age (r = − 0.122, p = 0.01; r = − 0.128; p = 0.007). After the third vaccination titers of nAb against wild-type SARS-CoV-2 were significantly correlated with age (r = − 0.245, p = 0.046; Fig. 7B), while BAU levels and titers against Omicron subvariants showed a non-significant trend (BAU: r = − 0.234, p = 0.057; BA.1: r = − 0.23, p = 0.074; BA.4/5: r = − 0.21, p = 0.096; BQ.1.1: r = − 0.11, p = 0.4; Fig. 7A/C). Antibody titers also showed a partially significant correlation with patients BMI (body mass index; BAU: r = 0.09, p = 0.064; nAb: r = 0.123; p = 0.011; ND50: r = 0.143, p = 0.038; ND50 BA.4/5: r = 0.322, p = 0.011). No sex-specific differences were detected (BAU: p = 0.25; nAb: 0.993; ND50: p = 0.83).
Use of non-steroidal anti-inflammatory drugs (NSAID), paracetamol or dexamethasone on the day of or following the initial or second vaccination neither influenced anti-SARS-CoV-2 IgG nor nAb levels.
Interestingly, patients who had an influenza vaccination within five years prior displayed a significantly higher SARS-CoV-2 IgG anti-S antibody and nAb response following their initial vaccination (114.3 vs. 25.1 BAU/ml, p = 0.05; 28% vs. 8%, p = 0.035), but not after third vaccination or any other timepoint of the study. Prior pneumococcal vaccination did not influence antibody response.
Out of those patients who were evaluated post-booster vaccination, six (6/61, 10%) did not exhibit detectable ND50 titers. These patients had significantly lower neutralization capacities against parental SARS-CoV-2 (39% vs. 73%; p = 0.001) and lower BAU titers, although slightly not significant (p = 0.064). Their characteristics are displayed in Table 3.
Discussion
This study presents data on humoral immune response in a representative cohort of cancer patients vulnerable to SARS-CoV-2 infections and severe COVID-19, including antibody analysis and neutralization titers of the currently most important and emerging variants of concern (i.e., Omicron BA.1, BA.4/5 and BQ.1.1). Most of the current studies on antibody response against the Omicron VOC focus on specific subgroups (e.g., lung cancer patients Mack et al. 2022; Valanparambil et al. 2022), patients with B cell malignancies (Greenberger et al. 2021) or patients after allogeneic hematopoietic stem cell transplant (Canti et al. 2022; Watanabe et al. 2022). So far, there are only a few reports on the efficacy of a third vaccination comparing different malignancies and treatments (Lasagna et al. 2022; Shapiro et al. 2022a, b; Zeng et al. 2022), however, those demonstrate an increase in antibody titers and nAb against Omicron after booster vaccination (Fendler et al. 2022). Nonetheless, questions remain as some patients still exhibit low immune responses. Furthermore, the durability of immunization, especially against VOC, is still not clear, necessitating additional studies with regard to subvariants and the influence of various factors like applied vaccine, age, cancer type, treatments or sex.
Based on the results of the IgG antibody titers, our study provides longitudinal data demonstrating an adequate immune response in most patients with cancer in regards of antibody titers. However, while antibody levels correlated with the relevant outcome of neutralizing antibodies against parental SARS-CoV-2, a considerable number of patients exhibited low levels of nAb three months following initial vaccination. Those increased significantly following the third vaccination. This is in line with previous data on neutralizing antibodies against SARS-CoV-2 VOCs and the efficacy of the administration of a booster vaccine (Khan et al. 2022; Terada et al. 2022; Wagner et al. 2022). Rate of SARS-CoV-2 breakthrough infections in our cohort was low. However, those patients who contracted COVID-19 post-vaccination had significantly lower antibody and nAb titers prior to their infection, indicating both vaccine effectiveness in the remaining cohort as well as an association of reduced antibody responses with a higher risk of SARS-CoV-2 breakthrough infections. This is supported by recent data from Lee et al. and highlights the importance of additional vaccinations for groups at risk (Lee et al. 2022; Macrae et al. 2022). None of our patients with breakthrough infection experienced a severe course of COVID-19.
In addition, ND50 titers against the Omicron VOC BA.1 were minimal prior and increased significantly after the third vaccination. We demonstrated ND50 levels to be independent of SARS-CoV-2 IgG titers and nAb against the parental SARS-CoV-2 strain, except for post-third vaccination levels. However, a concerning number of patients in our cohort (10%) did not elicit detectable ND50 titers against BA.1 after booster vaccination, although BAU and nAb titers were high. Only a third of these patients had a hematological malignancy and were therefore considered severely immune impaired, the remaining patients had solid cancers and received conventional cytostatic chemotherapy or immunotherapy. Low or undetectable neutralization of Omicron BA.1 after anti-SARS-CoV-2 vaccination in cancer patients has previously been described (Garcia-Beltran et al. 2022; Terada et al. 2022). This highlights the Omicron VOC’s capability of immune evasion as well as the need for the administration of a booster vaccine in cancer patients to cope with this. Further research is required to explain impaired immune responses in oncological patients, especially with respect to evasive VOCs like Omicron and its subvariants (Valanparambil et al. 2022; Zeng et al. 2022).
To the authors’ knowledge, this is one of the first studies providing data on neutralization concerning the Omicron BQ.1.1 subvariant following the third vaccination in a cohort of cancer patients. ND50 titers against BQ.1.1 were significantly lower than those against BA.1 or BA.4/5, which is coherent with recent results by Ehmsen et al. (2023). Almost half of our evaluated patients did not elicit detectable titers. Together with this subvariant’s previously reported resilience against monoclonal antibodies, this finding highlights the considerable risk of this and potentially other emerging SARS-CoV-2 to cancer patients.
In accordance with previous findings, SARS-CoV-2 IgG and nAb levels decreased over time and were negatively correlated or associated with patients’ age, a hematological malignancy or treatment with B cell depleting therapy, respectively (Lim et al. 2022; Obeid et al. 2022; Shapiro et al. 2022a, b). Choice of mRNA booster vaccination or sex did not influence the observed immune response.
Interestingly, we found a prior influenza vaccination to be significantly correlated to increased levels of nAb following the initial SARS-CoV-2 vaccination in cancer patients. Potentially, this could be explained by cross-immunity or trained immunity, as previously reported for SARS-CoV-2 infected patients with prior influenza vaccination (Debisarun et al. 2021; Poniedzialek et al. 2022). More research is necessary to fully elucidate this aspect, as this might indicate an interesting mechanism to increase immune response in cancer patients or potentially other patients with an impaired immune system.
Some limitations need to be considered: T-cell responses were not analyzed and, albeit data on healthy groups and reduced immunogenicity or efficacy of anti-SARS-CoV-2 vaccination in cancer patients has been previously reported (Garcia-Beltran et al. 2022; Gong et al. 2022; Ozbay Kurt et al. 2022; Pajon et al. 2022; Valanparambil et al. 2022), the study lacks a healthy control group.
In conclusion, our research provides extensive longitudinal data on cancer patient’s immune response and associated clinical factors following SARS-CoV-2 vaccination with a focus on the third (“booster”) vaccination. These are also among the first data on protection against the emerging Omicron subvariant BQ.1.1 in a representative cohort of cancer patients. Since a considerable number of patients did not display an adequate immune response, our results support continued efforts to apply booster and possibly repeated vaccines as well as reasonable caution. Further research is warranted to identify underlying mechanisms to overcome impaired immune response.
Data availability
The data used and analyzed during this study are available from the corresponding author upon reasonable request.
References
Addeo A, Shah PK, Bordry N, Hudson RD, Albracht B, Di Marco M, Kaklamani V, Dietrich PY, Taylor BS, Simand PF, Patel D, Wang J, Labidi-Galy I, Fertani S, Leach RJ, Sandoval J, Mesa R, Lathrop K, Mach N, Shah DP (2021) Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 39(8):1091–1098
Arora P, Kempf A, Nehlmeier I, Schulz SR, Cossmann A, Stankov MV, Jack HM, Behrens GMN, Pohlmann S, Hoffmann M (2022a) Augmented neutralisation resistance of emerging omicron subvariants BA2.12.1, BA.4, and BA.5. Lancet Infect Dis 22(8):1117–1118
Arora P, Kempf A, Nehlmeier I, Schulz SR, Jack HM, Pohlmann S, Hoffmann M (2022b) Omicron sublineage BQ.1.1 resistance to monoclonal antibodies. Lancet Infect Dis 23(1):22–23
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, Alroy-Preis S, Ash N, Huppert A, Milo R (2022) Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N Engl J Med 386:1712–1720
Canti L, Arien KK, Desombere I, Humblet-Baron S, Pannus P, Heyndrickx L, Henry A, Servais S, Willems E, Ehx G, Goriely S, Seidel L, Michiels J, Willems B, Goossens ME, Beguin Y, Marchant A, Baron F (2022) Antibody response against SARS-CoV-2 Delta and Omicron variants after third-dose BNT162b2 vaccination in allo-HCT recipients. Cancer Cell 40(4):335–337
Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, Huang W, Li Q, Wang P, An R, Wang J, Wang Y, Niu X, Yang S, Liang H, Sun H, Li T, Yu Y, Cui Q, Liu S, Yang X, Du S, Zhang Z, Hao X, Shao F, Jin R, Wang X, Xiao J, Wang Y, Xie XS (2022) Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602(7898):657–663
Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoako DG, Karim F, Bernstein M, Lustig G, Archary D, Smith M, Ganga Y, Jule Z, Reedoy K, Hwa SH, Giandhari J, Blackburn JM, Gosnell BI, Abdool Karim SS, Hanekom W, Ngs SA, Team C-K, von Gottberg A, Bhiman JN, Lessells RJ, Moosa MS, Davenport MP, de Oliveira T, Moore PL, Sigal A (2022) Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602(7898):654–656
Chang A, Akhtar A, Linderman SL, Lai L, Orellana-Noia VM, Valanparambil R, Ahmed H, Zarnitsyna VI, McCook-Veal AA, Switchenko JM, Koff JL, Blum KA, Ayers AA, O'Leary CB, Churnetski MC, Sulaiman S, Kives M, Sheng P, Davis CW, Nooka AK, Antia R, Dhodapkar MV, Suthar MS, Cohen JB, Ahmed R (2022) Humoral responses against SARS-CoV-2 and variants of concern after mRNA vaccines in patients with non-hodgkin lymphoma and chronic lymphocytic leukemia. J Clin Oncol 40(26):3020–3031
Davis-Gardner ME, Lai L, Wali B, Samaha H, Solis D, Lee M, Porter-Morrison A, Hentenaar IT, Yamamoto F, Godbole S, Douek DC, Lee FE, Rouphael N, Moreno A, Pinsky BA, Suthar MS (2022) mRNA bivalent booster enhances neutralization against BA.2.75.2 and BQ.1.1. bioRxiv
Debisarun PA, Gossling KL, Bulut O, Kilic G, Zoodsma M, Liu Z, Oldenburg M, Ruchel N, Zhang B, Xu CJ, Struycken P, Koeken V, Dominguez-Andres J, Moorlag S, Taks E, Ostermann PN, Muller L, Schaal H, Adams O, Borkhardt A, Ten Oever J, van Crevel R, Li Y, Netea MG (2021) Induction of trained immunity by influenza vaccination - impact on COVID-19. PLoS Pathog 17(10):e1009928
Edara VV, Manning KE, Ellis M, Lai L, Moore KM, Foster SL, Floyd K, Davis-Gardner ME, Mantus G, Nyhoff LE, Bechnak S, Alaaeddine G, Naji A, Samaha H, Lee M, Bristow L, Gagne M, Roberts-Torres J, Henry AR, Godbole S, Grakoui A, Saxton M, Piantadosi A, Waggoner JJ, Douek DC, Rouphael N, Wrammert J, Suthar MS (2022) mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep Med 3(2):100529
Ehmsen S, Pedersen RM, Bang LL, Asmussen A, Kragh A, Holm DK, Sydenham TV, Jensen TG, Jeppesen SS, Frederiksen H, Andersen TE, Ditzel HJ (2023) BQ.1.1, XBB.1, and XBB.1.5 neutralization after bivalent mRNA COVID-19 booster in patients with cancer. Cancer Cell 41(4):649–650
Fendler A, Shepherd STC, Au L, Wilkinson KA, Wu M, Byrne F, Cerrone M, Schmitt AM, Joharatnam-Hogan N, Shum B, Tippu Z, Rzeniewicz K, Boos LA, Harvey R, Carlyle E, Edmonds K, Del Rosario L, Sarker S, Lingard K, Mangwende M, Holt L, Ahmod H, Korteweg J, Foley T, Bazin J, Gordon W, Barber T, Emslie-Henry A, Xie W, Gerard CL, Deng D, Wall EC, Agua-Doce A, Namjou S, Caidan S, Gavrielides M, MacRae JI, Kelly G, Peat K, Kelly D, Murra A, Kelly K, O’Flaherty M, Dowdie L, Ash N, Gronthoud F, Shea RL, Gardner G, Murray D, Kinnaird F, Cui W, Pascual J, Rodney S, Mencel J, Curtis O, Stephenson C, Robinson A, Oza B, Farag S, Leslie I, Rogiers A, Iyengar S, Ethell M, Messiou C, Cunningham D, Chau I, Starling N, Turner N, Welsh L, van As N, Jones RL, Droney J, Banerjee S, Tatham KC, O’Brien M, Harrington K, Bhide S, Okines A, Reid A, Young K, Furness AJS, Pickering L, Swanton C, Crick C-C, Gandhi S, Gamblin S, Bauer DLV, Kassiotis G, Kumar S, Yousaf N, Jhanji S, Nicholson E, Howell M, Walker S, Wilkinson RJ, Larkin J, Turajlic S, Consortium C (2021) Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer 2(12):1305–1320
Fendler A, Shepherd STC, Au L, Wu M, Harvey R, Schmitt AM, Tippu Z, Shum B, Farag S, Rogiers A, Carlyle E, Edmonds K, Del Rosario L, Lingard K, Mangwende M, Holt L, Ahmod H, Korteweg J, Foley T, Barber T, Emslie-Henry A, Caulfield-Lynch N, Byrne F, Deng D, Kjaer S, Song OR, Queval C, Kavanagh C, Wall EC, Carr EJ, Caidan S, Gavrielides M, MacRae JI, Kelly G, Peat K, Kelly D, Murra A, Kelly K, O’Flaherty M, Shea RL, Gardner G, Murray D, Yousaf N, Jhanji S, Tatham K, Cunningham D, Van As N, Young K, Furness AJS, Pickering L, Beale R, Swanton C, Gandhi S, Gamblin S, Bauer DLV, Kassiotis G, Howell M, Nicholson E, Walker S, Larkin J, Turajlic S, Consortium C (2022) Omicron neutralising antibodies after third COVID-19 vaccine dose in patients with cancer. Lancet 399(10328):905–907
Garcia-Beltran WF, St-Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, Feldman J, Roederer AL, Gregory DJ, Poznansky MC, Schmidt AG, Iafrate AJ, Naranbhai V, Balazs AB (2022) mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185(3):457–466
Giuliano AR, Lancet JE, Pilon-Thomas S, Dong N, Jain AG, Tan E, Ball S, Tworoger SS, Siegel EM, Whiting J, Mo Q, Cubitt CL, Dukes CW, Hensel JA, Keenan RJ, Hwu P (2022) Evaluation of antibody response to SARS-CoV-2 mRNA-1273 vaccination in patients with cancer in Florida. JAMA Oncol 8:748–754
Gong IY, Vijenthira A, Powis M, Calzavara A, Patrikar A, Sutradhar R, Hicks LK, Wilton D, Singh S, Krzyzanowska MK, Cheung MC (2022) Association of COVID-19 vaccination with breakthrough infections and complications in patients with cancer. JAMA Oncol 9(3):386–394
Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL (2021) Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell 39(10):1297–1299
Hoffmann M, Kruger N, Schulz S, Cossmann A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer AS, Winkler MS, Lier M, Dopfer-Jablonka A, Jack HM, Behrens GMN, Pohlmann S (2022) The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 185(3):447–456
Khan QJ, Bivona CR, Martin GA, Zhang J, Liu B, He J, Li KH, Nelson M, Williamson S, Doolittle GC, Sun W, Mudaranthakam DP, Streeter NR, McGuirk JP, Al-Rajabi R, Hoffmann M, Kasi A, Parikh RA, Zhong C, Mitchell L, Pessetto ZY, Pathak H, Ghosh A, LaFaver S, Sharma P, Godwin AK (2022) Evaluation of the durability of the immune humoral response to COVID-19 vaccines in patients with cancer undergoing treatment or who received a stem cell transplant. JAMA Oncol 8(7):1053–1058
Koch J, Vygen-Bonnet S, Bogdan C, Burchard G, Garbe E, Heininger U, Hummers E, Kling K, von Kries R, Ledig T, Littmann M, Meerpohl J, Mertens T, Meyer H, Perumal N, Röbl-Mathieu M, van der Sande M, Schönfeld V, Steffen A, Terhardt M, Überla K, Wichmann O, Wicker S, Wiedermann-Schmidt U, Widders G, Zepp F (2022) STIKO-Empfehlung zur 2. COVID-19-Auffrischimpfung mit einem mRNA- Impfstoff für besonders gesundheitlich gefährdete bzw. exponierte Personengruppen und die dazugehörige wissenschaftliche Begründung. Epid Bull 7:41–57
Kurhade C, Zou J, Xia H, Liu M, Chang HC, Ren P, Xie X, Shi PY (2022b) Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1, and XBB.1 by parental mRNA vaccine or a BA.5-bivalent booster. Nat Med 29(2):344–347
Lasagna A, Bergami F, Lilleri D, Percivalle E, Quaccini M, Alessio N, Comolli G, Sarasini A, Sammartino JC, Ferrari A, Arena F, Secondino S, Cicognini D, Schiavo R, Lo Cascio G, Cavanna L, Baldanti F, Pedrazzoli P, Cassaniti I (2022) Immunogenicity and safety after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with solid tumors on active treatment: a prospective cohort study. ESMO Open 7(2):100458
Lee LYW, Cazier JB, Starkey T, Briggs SEW, Arnold R, Bisht V, Booth S, Campton NA, Cheng VWT, Collins G, Curley HM, Earwaker P, Fittall MW, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJX, Lee RJ, Lee SM, Mckenzie H, Middleton CP, Murugaesu N, Newsom-Davis T, Olsson-Brown AC, Palles C, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Topping O, Turnbull CD, Várnai C, Briggs ADM, Middleton G, Kerr R, Team UCCMP (2020) COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 21(10):1309–1316
Lee LYW, Tilby M, Starkey T, Ionescu MC, Burnett A, Hattersley R, Khan S, Little M, Liu JKH, Platt JR, Tripathy A, Watts I, Williams ST, Appanna N, Al-Hajji Y, Barnard M, Benny L, Buckley A, Cattell E, Cheng V, Clark J, Eastlake L, Gerrand K, Ghafoor Q, Grumett S, Harper-Wynne C, Kahn R, Lee AJX, Lydon A, McKenzie H, Panneerselvam H, Pascoe J, Patel G, Patel V, Potter V, Randle A, Rigg AS, Robinson T, Roylance R, Roques T, Rozmanowski S, Roux RL, Shah K, Sintler M, Taylor H, Tillett T, Tuthill M, Williams S, Beggs A, Iveson T, Lee SM, Middleton G, Middleton M, Protheroe AS, Fittall MW, Fowler T, Johnson P, Progamme UCC (2022) Association of SARS-CoV-2 spike protein antibody vaccine response with infection severity in patients with cancer: a national COVID cancer cross-sectional evaluation. JAMA Oncol 9(2):188–196
Lim SH, Stuart B, Joseph-Pietras D, Johnson M, Campbell N, Kelly A, Jeffrey D, Turaj AH, Rolfvondenbaumen K, Galloway C, Wynn T, Coleman AR, Ward B, Long K, Coleman H, Mundy C, Bates AT, Ayres D, Lown R, Falconer J, Brake O, Batchelor J, Willimott V, Bowzyk Al-Naeeb A, Robinson L, O’Callaghan A, Collins GP, Menne T, Faust SN, Fox CP, Ahearne M, Johnson PWM, Davies AJ, Goldblatt D (2022) Immune responses against SARS-CoV-2 variants after two and three doses of vaccine in B-cell malignancies: UK PROSECO study. Nat Cancer 3:552–564
Mack PC, Gomez JE, Rodilla AM, Carreno JM, Hsu CY, Rolfo C, Meshulami N, Moore A, Brody RI, King JC, Treatman J, Lee S, Raskin A, Srivastava K, Gleason CR, de Miguel-Perez D, Group PPS, Tcheou J, Bielak D, Acharya R, Gerber DE, Rohs N, Henschke CI, Yankelevitz DF, Simon V, Minna JD, Bunn PA Jr, Garcia-Sastre A, Krammer F, Shyr Y, Hirsch FR (2022) Longitudinal COVID-19-vaccination-induced antibody responses and Omicron neutralization in patients with lung cancer. Cancer Cell 40:575–577
Macrae K, Martinez-Cajas J, Bessai K, Abdulhamed A, Gong Y (2022) Quantitative analysis of SARS-CoV-2 antibody levels in cancer patients post three doses of immunization and prior to breakthrough COVID-19 infections. Curr Oncol 29(10):7059–7071
Nooka AK, Shanmugasundaram U, Cheedarla N, Verkerke H, Edara VV, Valanparambil R, Kaufman JL, Hofmeister CC, Joseph NS, Lonial S, Azeem M, Manalo J, Switchenko JM, Chang A, Linderman SL, Roback JD, Dhodapkar KM, Ahmed R, Suthar MS, Neish AS, Dhodapkar MV (2022) Determinants of neutralizing antibody response after SARS CoV-2 vaccination in patients with myeloma. J Clin Oncol 40(26):3057–3064
Obeid M, Suffiotti M, Pellaton C, Bouchaab H, Cairoli A, Salvadé V, Stevenel C, Hottinger R, Pythoud C, Coutechier L, Molinari L, Trono D, Ribi C, Gottardo R, Fenwick C, Pascual M, Duchosal MA, Peters S, Pantaleo G (2022) Humoral responses against variants of concern by COVID-19 mRNA vaccines in immunocompromised patients. JAMA Oncol 8:e220446
Overheu O, Lendowski S, Quast DR, Marheinecke CS, Kourti E, Lugnier C, Andreica I, Kiltz U, Pfaender S, Reinacher-Schick A (2022c) Attitude towards and perception of individual safety after SARS-CoV-2 vaccination among German cancer patients. J Cancer Res Clin Oncol 149(5):1985–1992
Ozbay Kurt FG, Lepper A, Gerhards C, Roemer M, Lasser S, Arkhypov I, Bitsch R, Bugert P, Altevogt P, Gouttefangeas C, Neumaier M, Utikal J, Umansky V (2022) Booster dose of mRNA vaccine augments waning T cell and antibody responses against SARS-CoV-2. Front Immunol 13:1012526
Pajon R, Doria-Rose NA, Shen X, Schmidt SD, O’Dell S, McDanal C, Feng W, Tong J, Eaton A, Maglinao M, Tang H, Manning KE, Edara VV, Lai L, Ellis M, Moore KM, Floyd K, Foster SL, Posavad CM, Atmar RL, Lyke KE, Zhou T, Wang L, Zhang Y, Gaudinski MR, Black WP, Gordon I, Guech M, Ledgerwood JE, Misasi JN, Widge A, Sullivan NJ, Roberts PC, Beigel JH, Korber B, Baden LR, El Sahly H, Chalkias S, Zhou H, Feng J, Girard B, Das R, Aunins A, Edwards DK, Suthar MS, Mascola JR, Montefiori DC (2022) SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 386(11):1088–1091
Planas D, Bruel T, Staropoli I, Guivel-Benhassine F, Porrot F, Maes P, Grzelak L, Prot M, Mougari S, Planchais C, Puech J, Saliba M, Sahraoui R, Femy F, Morel N, Dufloo J, Sanjuan R, Mouquet H, Andre E, Hocqueloux L, Simon-Loriere E, Veyer D, Prazuck T, Pere H, Schwartz O (2022). Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies. bioRxiv
Poniedzialek B, Hallmann E, Sikora D, Szymanski K, Kondratiuk K, Zurawski J, Rzymski P, Brydak L (2022) Relationship between humoral response in COVID-19 and seasonal influenza vaccination. Vaccines (basel) 10(10):1621
Qu P, Evans JP, Faraone JN, Zheng YM, Carlin C, Anghelina M, Stevens P, Fernandez S, Jones D, Lozanski G, Panchal A, Saif LJ, Oltz EM, Xu K, Gumina RJ, Liu SL (2022) Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe 31(1):9–17.e3
Rüthrich MM, Giessen-Jung C, Borgmann S, Classen AY, Dolff S, Grüner B, Hanses F, Isberner N, Köhler P, Lanznaster J, Merle U, Nadalin S, Piepel C, Schneider J, Schons M, Strauss R, Tometten L, Vehreschild JJ, von Lilienfeld-Toal M, Beutel G, Wille K, Group LS (2021) COVID-19 in cancer patients: clinical characteristics and outcome-an analysis of the LEOSS registry. Ann Hematol 100(2):383–393
Shapiro JR, Sitaras I, Park HS, Aytenfisu TY, Caputo C, Li M, Lee J, Johnston TS, Li H, Wouters C, Hauk P, Jacobsen H, Li Y, Abrams E, Yoon S, Kocot AJ, Yang T, Huang Y, Cramer SM, Betenbaugh MJ, Debes AK, Morgan R, Milstone AM, Karaba AH, Pekosz A, Leng SX, Klein SL (2022a) Association of frailty, age, and biological sex with severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine-induced immunity in older adults. Clin Infect Dis 75(Supplement_1):S61–S71
Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, Quinn R, Bhagat TD, Choudhary GS, McCort M, Sica RA, Goldfinger M, Goel S, Anampa JD, Levitz D, Fromowitz A, Shah AP, Sklow C, Alfieri G, Racine A, Wolgast L, Greenberger L, Verma A, Halmos B (2022b) Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell 40(1):3–5
Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, Peyton KL, Uhrlaub JL, Ripperger TJ, Jergovic M, Dalgai S, Wolf A, Whitmer R, Hammad H, Carrier A, Scott AJ, Nikolich-Zugich J, Worobey M, Sprissler R, Dake M, LaFleur BJ, Bhattacharya D (2021) Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med 27(11):2002–2011
Terada M, Kondo N, Wanifuchi-Endo Y, Fujita T, Asano T, Hisada T, Uemoto Y, Akiko K, Yamanaka N, Sugiura H, Mita K, Wada A, Takahashi E, Saito K, Yoshioka R, Toyama T (2022) Efficacy and impact of SARS-CoV-2 vaccination on cancer treatment for breast cancer patients: a multi-center prospective observational study. Breast Cancer Res Treat 195(3):311–323
Valanparambil RM, Carlisle J, Linderman SL, Akthar A, Millett RL, Lai L, Chang A, McCook-Veal AA, Switchenko J, Nasti TH, Saini M, Wieland A, Manning KE, Ellis M, Moore KM, Foster SL, Floyd K, Davis-Gardner ME, Edara VV, Patel M, Steur C, Nooka AK, Green F, Johns MA, O'Brein F, Shanmugasundaram U, Zarnitsyna VI, Ahmed H, Nyhoff LE, Mantus G, Garett M, Edupuganti S, Behra M, Antia R, Wrammert J, Suthar MS, Dhodapkar MV, Ramalingam S, Ahmed R (2022) Antibody response to COVID-19 mRNA vaccine in patients with lung cancer after primary immunization and booster: reactivity to the SARS-CoV-2 WT virus and omicron variant. J Clin Oncol 40(33):3808–3816
Wagner A, Garner-Spitzer E, Schotta AM, Orola M, Wessely A, Zwazl I, Ohradanova-Repic A, Weseslindtner L, Tajti G, Gebetsberger L, Kratzer B, Tomosel E, Kutschera M, Tobudic S, Pickl WF, Kundi M, Stockinger H, Novacek G, Reinisch W, Zielinski C, Wiedermann U (2022) SARS-CoV-2-mRNA booster vaccination reverses non-responsiveness and early antibody waning in immunocompromised patients—a phase four study comparing immune responses in patients with solid cancers, multiple myeloma and inflammatory bowel disease. Front Immunol 13:889138
Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, Bowen AD, Liu M, Wang M, Yu J, Valdez R, Lauring AS, Sheng Z, Wang HH, Gordon A, Liu L, Ho DD (2022d) Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186(2):279–286.e8
Watanabe M, Yakushijin K, Funakoshi Y, Ohji G, Ichikawa H, Sakai H, Hojo W, Saeki M, Hirakawa Y, Matsumoto S, Sakai R, Nagao S, Kitao A, Miyata Y, Koyama T, Saito Y, Kawamoto S, Yamamoto K, Ito M, Murayama T, Matsuoka H, Minami H (2022) A third dose COVID-19 vaccination in allogeneic hematopoietic stem cell transplantation patients. Vaccines 10(11):1830
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821):430–436
Zeng C, Evans JP, Chakravarthy K, Qu P, Reisinger S, Song NJ, Rubinstein MP, Shields PG, Li Z, Liu SL (2022) COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell 40(2):117–119
Zettl F, Meister TL, Vollmer T, Fischer B, Steinmann J, Krawczyk A, V’Kovski P, Todt D, Steinmann E, Pfaender S, Zimmer G (2020) Rapid quantification of SARS-CoV-2-neutralizing antibodies using propagation-defective vesicular stomatitis virus pseudotypes. Vaccines (basel) 8(3):386
Acknowledgements
We thank Gert Zimmer, Institute for Virology and Immunology, Switzerland and Department of Infectious Diseases and Pathobiology (DIP), Vetsuisse Faculty, University of Bern, Switzerland, and Stefan Pöhlmann and Markus Hoffmann, Infection Biology Unit, German Primate Center – Leibniz Institute for Primate Research, Göttingen, Germany, Faculty of Biology and Psychology, Georg-August-University Göttingen, Göttingen, Germany, for providing the VSV-system and SARS-CoV-2 plasmids.
Funding
Open Access funding enabled and organized by Projekt DEAL. No external funding was received.
Author information
Authors and Affiliations
Contributions
OO and SL collected the data and samples. DK, EVB, JS, ES and SP conducted the laboratory analyses. OO and DQ analyzed the data. OO drafted the first manuscript. OO, AR-S and SP supervised the study. All authors contributed to the conceptional design of the study. All authors read, corrected, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial conflicts of interest with respect to this study to disclose.
Ethics approval
The study was approved by the Ethics Committee of the Medical Faculty, Ruhr University Bochum (reference number 20-6953-bio and 21-7351) and conducted in accordance with the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Overheu, O., Lendowski, S., Quast, D.R. et al. Longitudinal data on humoral response and neutralizing antibodies against SARS-CoV-2 Omicron BA.1 and subvariants BA.4/5 and BQ.1.1 after COVID-19 vaccination in cancer patients. J Cancer Res Clin Oncol 149, 10633–10644 (2023). https://doi.org/10.1007/s00432-023-04961-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04961-2