Abstract
Purpose
An increasing infiltration of FoxP3-positive T-regs is associated with a higher grade of cervical intraepithelial neoplasia. The T-reg-recruiting chemokine CCL22 is expressed in various tumour entities. Aim of our study was to investigate the role of CCL22 in the progression and regression of cervical intraepithelial neoplasias, especially in patients with intermediate cervical intraepithelial neoplasias (CIN II). Furthermore, our aim was to characterize the CCL22-producing cells and explore the role of innate immunity in the process of cells recruitment.
Methods
CCL22 expression was analyzed immunohistochemically in 169 patient samples. The immunoreactive score as well as the median numbers of positive cells were calculated in each slide and correlated with the histological CIN grade and FoxP3 expression. Additionally, CD68/CCL22 as well as CD68/PPARγ and CD68/FoxP3 expression were examined by double immunofluorescence. Statistical analysis was performed by SPSS 26.
Results
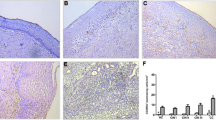
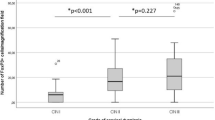
A significantly higher expression of epithelial CCL22 in CIN II with progression in comparison to CIN II with regression (p = 0.006) could be detected. CCL22 was correlated with FoxP3 (Spearman’s Rho: 0.308; p < 0.01). In 88%, CCL22-positive cells were positive for CD68, and 71% of CD68-positive macrophages expressed PPARγ. Colocalization of CD68 and FoxP3 was detected in 12%.
Conclusion
We could demonstrate that increased expression of CCL22, mainly produced by macrophages, correlates with elevated potential of malignancy. CCL22 expression could act as a predictor for regression and progression in cervical intraepithelial neoplasia, and it may help in the decision process regarding surgical treatment versus watchful waiting strategy in order to prevent conisation-associated risks. Furthermore, our findings support the potential of CCL22-producing cells as a target for immune therapy in cervical cancer patients.







Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the correspondent author on reasonable request.
Abbreviations
- CCL:
-
C–C-chemokine ligand
- CCR:
-
C–C-chemokine receptor
- CD:
-
Cluster differentiation
- CIN:
-
Cervical intraepithelial neoplasia
- CTLA4:
-
Cytotoxic T-lymphocyte-associated protein 4
- Cy-2, Cy-3:
-
Cyanine-2,-3
- Fig:
-
Figure
- FoxP3:
-
Forkhead box protein 3
- GITR:
-
Glucocorticoid-induced TNF-Receptor
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- HB-EGF:
-
Heparin-binding epidermal growth factor like growth factor
- HER2:
-
Human epidermal growth factor receptor 2
- HLA-DR:
-
Human leukocyte antigene
- HSIL:
-
High grade squamous intraepithelial lesion
- IL:
-
Interleukine
- IRS-Score:
-
Immunoreactive score
- LAG3:
-
Lymphocyte activating 3
- LEEP/LEETZ:
-
Loop electrosurgical procedure/large loop excision of the transformation zone
- LSIL:
-
Low grade squamous intraepithelial lesion
- PBS:
-
Phosphate buffered saline
- PD-L1:
-
Programmed death ligand 1
- PGE-2:
-
Prostaglandine E
- PPAR:
-
Peroxisome-proliferator-activated receptor
- SKP2:
-
S-Phase kinase associated protein 2
- Smad2/3:
-
MAD (mothers against decapentaplegic)/ sma (small body size)
- TCR:
-
T-cell receptor
- TGF:
-
Transforming growth factor
- TLR8:
-
Toll-like receptor 8
- Treg:
-
Regulatory T-cell
- VEGF:
-
Vascular endothelial growth factor
References
Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, Prendiville W, Paraskevaidis E (2008) Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. Bmj 337:a1284. https://doi.org/10.1136/bmj.a1284
Arck P, Toth B, Pestka A, Jeschke U (2010) Nuclear receptors of the peroxisome proliferator-activated receptor (PPAR) family in gestational diabetes: from animal models to clinical trials1. Biol Reprod 83:168–176. https://doi.org/10.1095/biolreprod.110.083550
Arends MJ, Buckley CH, Wells M (1998) Aetiology, pathogenesis, and pathology of cervical neoplasia. J Clin Pathol 51:96–103. https://doi.org/10.1136/jcp.51.2.96
Betjes MG, Haks MC, Tuk CW, Beelen RH (1991) Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-term culture. Immunobiology 183:79–87. https://doi.org/10.1016/S0171-2985(11)80187-7
Bolpetti A, Silva JS, Villa LL, Lepique AP (2010) Interleukin-10 production by tumor infiltrating macrophages plays a role in human papillomavirus 16 tumor growth. BMC Immunol 11:27. https://doi.org/10.1186/1471-2172-11-27
Boyer, S. N., Wazer, D. E., Band, V. (1996) E7 Protein of Human Papilloma Virus-16 Induces Degradation of Retinoblastoma Protein through the Ubiquitin-Proteasome Pathway. Cancer research, 56, 4620–4624. https://cancerres.aacrjournals.org/content/canres/56/20/4620.full.pdf
Castellsagué X, Bosch FX, Muñoz N (2002) Environmental co-factors in HPV carcinogenesis. Virus Res 89:191–199. https://doi.org/10.1016/s0168-1702(02)00188-0
Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, Nevins JR (1992) Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Procee N Acade Sci United States Am 89:4549–4553. https://doi.org/10.1073/pnas.89.10.4549
Chen X-J, Han L-F, Wu X-G, Wei W-F, Wu L-F, Yi H-Y, Yan R-M, Bai X-Y, Zhong M, Yu Y-H, Liang L, Wang W (2017) Clinical significance of CD163+ and CD68+ tumor-associated macrophages in high-risk HPV-related cervical cancer. J Cancer 8:3868–3875
Curiel TJ (2008) Regulatory T cells and treatment of cancer. Curr Opin Immunol 20:241–6. https://doi.org/10.1016/j.coi.2008.04.008
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949. https://doi.org/10.1038/nm1093
Davidson B, Goldberg I, Kopolovic J (1997) Inflammatory response in cervicallntraepithelial neoplasia and squamous cell carcinoma of the uterine cervix. Pathology - Res Practice 193:491–495. https://doi.org/10.1016/s0344-0338(97)80102-1
Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K (2009) Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58:2574–2582. https://doi.org/10.2337/db08-1475
Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW (1997) Human macrophage–derived Chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med 185:1595–1604. https://doi.org/10.1084/jem.185.9.1595
Gupta S, Takhar PPS, Degenkolbe R, Heng Koh C, Zimmermann H, Maolin Yang C, Guan Sim K, Hong Hsu S, Bernard HU (2003) The human papillomavirus type 11 and 16 E6 proteins modulate the cell-cycle regulator and transcription cofactor TRIP-Br 1. Virology 317:155–164
Hartwig T, Montinaro A, Von Karstedt S, Sevko A, Surinova S, Chakravarthy A, Taraborrelli L, Draber P, Lafont E, Arce Vargas F, El-Bahrawy MA, Quezada SA, Walczak H (2017) The TRAIL-induced cancer secretome promotes a tumor-supportive immune microenvironment via CCR2. Mol Cell 65:730-742.e5. https://doi.org/10.1016/j.molcel.2017.01.021
Heublein S, Vrekoussis T, Kuhn C, Friese K, Makrigiannakis A, Mayr D, Lenhard M, Jeschke U (2013) Inducers of G-protein coupled estrogen receptor (GPER) in endometriosis: potential implications for macrophages and follicle maturation. J Reprod Immunol 97:95–103. https://doi.org/10.1371/journal.pone.0071791
HEUSINKVELD, M., DE VOS VAN STEENWIJK, P. J., GOEDEMANS, R., RAMWADHDOEBE, T. H., GORTER, A., WELTERS, M. J. P., VAN HALL, T. & VAN DER BURG, S. H. , 2011 Heusinkveld, M., De Vos Van Steenwijk, P. J., Goedemans, R., Ramwadhdoebe, T. H., Gorter, A., Welters, M. J. P., Van Hall, T., Van Der Burg, S. H. (2011) M2 Macrophages Induced by Prostaglandin E<sub>2</sub> and IL-6 from Cervical Carcinoma Are Switched to Activated M1 Macrophages by CD4<sup>+</sup> Th1 Cells. The Journal of Immunology, 187, 1157-1165. https://doi.org/10.4049/jimmunol.1100889
Huang Y-H, Chang C-Y, Kuo Y-Z, Fang W-Y, Kao H-Y, Tsai S-T, Wu L-W (2019) Cancer-associated fibroblast-derived interleukin-1β activates protumor C-C motif chemokine ligand 22 signaling in head and neck cancer. Cancer Sci 110:2783–2793. https://doi.org/10.1111/cas.14135
Jiang S, Yang Y, Fang M, Li X, Yuan X, Yuan J (2016) Co-evolution of tumor-associated macrophages and tumor neo-vessels during cervical cancer invasion. Oncol Lett 12:2625–2631. https://doi.org/10.3892/ol.2016.5014
Kim J, Bae J-S (2016) Tumor-Associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm 2016:6058147. https://doi.org/10.1155/2016/6058147
Kolben TM, Rogatsch E, Vattai A, Hester A, Kuhn C, Schmoeckel E, Mahner S, Jeschke U, Kolben T (2018) PPARγ Expression Is diminished in macrophages of recurrent miscarriage placentas. Int J Mol Sci 19:1872. https://doi.org/10.3390/ijms19071872
Kyrgiou, M., Athanasiou, A., Paraskevaidi, M., Mitra, A., Kalliala, I., Martin-Hirsch, P., Arbyn, M., Bennett, P. Paraskevaidis, E. (2016) Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ (Clinical research ed.), 354, i3633-i3633. Kyrgiou, M., Athanasiou, A., Paraskevaidi, M., Mitra, A., Kalliala, I., Martin-Hirsch, P., Arbyn, M., Bennett, P., & Paraskevaidis, E. (2016). Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ (Clinical research ed.), 354, i3633-i3633. /https://doi.org/10.1136/bmj.i3633
Lepique AP, Daghastanli KRP, Cuccovia IM, Villa LL (2009) HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res 15:4391–4400. https://doi.org/10.1158/1078-0432.CCR-09-0489
LI, H., Sorenson, A. L., Poczobutt, J., Amin, J., Joyal, T., Sullivan, T., Crossno, J. T., JR., Weiser-Evans, M. C., Nemenoff, R. A. (2011) Activation of PPARγ in myeloid cells promotes lung cancer progression and metastasis. PLoS One, 6, e28133. https://doi.org/10.1371/journal.pone.0028133
Li YQ, Liu FF, Zhang XM, Guo XJ (2013) Tumor secretion of CCL22 activates intratumoral treg infiltration and is independent prognostic predictor of breast cancer. PLoS One 8:e76379. https://doi.org/10.1371/journal.pone.0076379
Liu X, Meng L, Chen L, Liang Y, Wang B, Shao Q, Wang H, Yang X (2020) IL-6 expression promoted by Poly(I:C) in cervical cancer cells regulates cytokine expression and recruitment of macrophages. J Cell Mol Med 24:2284–2293. https://doi.org/10.1111/jcmm.14911
Melichar B, Solichová D, Freedman RS (2006) Neopterin as an indicator of immune activation and prognosis in patients with gynecological malignancies. Int J Gynecol Cancer 16:240–252. https://doi.org/10.1111/j.1525-1438.2006.00294.x
Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8:138–140
Rigo A, Gottardi M, Zamò A, Mauri P, Boifacio M, Krampera M, Damiani E, Pizzolo G, Vinante F (2010) Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Molecular Cancer 9:273. https://doi.org/10.1186/1476-4598-9-273
Schaniel C, Pardali E, Sallusto F, Speletas M, Ruedl C, Shimizu T, Seidl T, Andersson J, Melchers F, Rolink AG, Sideras P (1998) Activated murine B Lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J Exp Med 188:451–463. https://doi.org/10.1084/jem.188.3.451
Scheffner, M., Werness, B. A., Huibregtse, J. M., Levine, A. J., Howley, P. M. (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell, 63, 1129–1136. Kyrgiou, M., Athanasiou, A., Paraskevaidi, M., Mitra, A., Kalliala, I., Martin-Hirsch, P., Arbyn, M., Bennett, P., & Paraskevaidis, E. (2016). Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ (Clinical research ed.), 354, i3633-i3633. /https://doi.org/10.1136/bmj.i3633
Scheu, S., Ali, S., Ruland, C., Arolt, V., Alferink, J. (2017) The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int J Mol Sci 18
Schiffman M, Wentzensen N (2013) Human papillomavirus Infection and the multistage carcinogenesis of cervical cancer. CEBP Focus 22:553–560. https://doi.org/10.1158/1055-9965.EPI-12-1406
Silver MI, Gage JC, Schiffman M, Fetterman B, Poitras NE, Lorey T, Cheung LC, Katki HA, Locke A, Kinney WK, Castle PE (2018) Clinical outcomes after conservative management of cervical intraepithelial neoplasia grade 2 (CIN2) in women ages 21–39 years. Cancer Prev Res 11:165–170. https://doi.org/10.1158/1940-6207.CAPR-17-0293
Slukvin II, Breburda EE, Golos TG (2004) Dynamic changes in primate endometrial leukocyte populations: differential distribution of macrophages and natural killer cells at the rhesus monkey implantation site and in early pregnancy. Placenta 25:297–307. https://doi.org/10.1016/j.placenta.2003.08.019
Stremmel C, Schuchert R, Wagner F, Thaler R, Weinberger T, Pick R, Mass E, Ishikawa-Ankerhold HC, Margraf A, Hutter S, Vagnozzi R, Klapproth S, Frampton J, Yona S, Scheiermann C, Molkentin JD, Jeschke U, Moser M, Sperandio M, Massberg S, Geissmann F, Schulz C (2018) Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat Commun 9:75
Tainio K, Athanasiou A, Tikkinen KAO, Aaltonen R, Cárdenas J, Glazer-Livson S, Jakobsson M, Joronen K, Kiviharju M, Louvanto K, Oksjoki S, Tähtinen R, Virtanen S, Nieminen P, Kyrgiou M, Kalliala I (2018) Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. The BMJ 360:k499. https://doi.org/10.1136/bmj.k499
Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312. https://doi.org/10.1146/annurev.biochem.77.061307.091829
Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Muñoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19. https://doi.org/10.1002/(sici)1096-9896(199909)189:1
Walentowicz-Sadlecka M, Koper A, Krystyna G, Koper K, Basta P, Mach P, Skret-Magierlo J, Dutsch-Wicherek M, Sikora J, Grabiec M, Kazmierczak W, Wicherek L (2013) The analysis of metallothionein immunoreactivity in stromal fibroblasts and macrophages in cases of uterine cervical carcinoma with respect to both the local and distant spread of the disease. Am J Reprod Immunol 70:253–261
Wang Q, Schmoeckel E, Kost BP, Kuhn C, Vattai A, Vilsmaier T, Mahner S, Mayr D, Jeschke U, Heidegger HH (2019) Higher CCL22+ cell Infiltration is associated with poor prognosis in cervical cancer patients. Cancers 11:2004. https://doi.org/10.3390/cancers11122004
Wong AA, Fuller J, Pabbaraju K, Wong S, Zahariadis G (2012) (2012) Comparison of the hybrid capture 2 and cobas 4800 tests for detection of high-risk human papillomavirus in specimens collected in preservcyt medium. J Clin Microbiol. 50(1):25–9. https://doi.org/10.1128/JCM.05400-11
World Health Organization. (2020) World health assembly adopts global strategy to accelerate cervical cancer elimination. Available: https://www.who.int/news/item/19-08-2020-world-health-assembly-adopts-global-strategy-to-accelerate-cervical-cancer-elimination [Accessed 26.10.2020].
WHO Guidelines for Treatment of Cervical Intraepithelial Neoplasia 2–3 and Adenocarcinoma in situ: Cryotherapy, Large Loop Excision of the Transformation Zone, and Cold Knife Conization. Geneva: World Health Organization; 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK206775/ (Retrieved: 01.08.2022)
Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, Drewlo S, Gomez-Lopez N (2016) An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J Immunol 196:2476–2491. https://doi.org/10.4049/jimmunol.1502055
Yashiro T, Nakano S, Nomura K, Uchida Y, Kasakura K, Nishiyama C (2019) A transcription factor PU1 is critical for Ccl22 gene expression in dendritic cells and macrophages. Sci Rep 9:1161
Zhao M, Li Y, Wei X, Zhang Q, Jia H, Quan S, Cao D, Wang L, Yang T, Zhao J, Pei M, Tian S, Yu Y, Guo Y, Yang X (2017) Negative immune factors might predominate local tumor immune status and promote carcinogenesis in cervical carcinoma. Virol J 14:5
Zhou M, Bracci PM, McCoy LS, Hsuang G, Wiemels JL, Rice T, Zheng S, Kelsey KT, Wrensch MR, Wiencke JK (2015) Serum macrophage-derived chemokine/CCL22 levels are associated with glioma risk, CD4 T cell lymphopenia and survival time. Int J Cancer 137:826–836. https://doi.org/10.1002/ijc.29441
Acknowledgements
We thank Christina Kuhn, Andrea Sendelhofert, and Anja Heier for their excellent technical assistance.
Funding
This study was funded by the Friedrich-Baur Foundation, LMU Munich.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by NK, AV, TK and LH. The first draft of the manuscript was written by NK, AV and TK and all authors commented on previous versions of the manuscript. All authors analysed and interpreted the data and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Sven Mahner—Research funding, advisory board, honorary or travel expenses: AbbVie, AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Hubro, Medac, MSD, Novartis, Nykode, Olympus, PharmaMar, Pfizer, Roche, Sensor Kinesis, Teva, Tesaro.
Ethical approval
All patients gave informed consent prior to study participation. All procedures were performed according to the ethical standards of the institutional and/or national research committee and with the declaration of Helsinki of 1964 and its later amendments. This study was approved by the local ethics committee of the Ludwig-Maximilians-University Munich, Germany (167–14).
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vattai, A., Kremer, N., Meister, S. et al. Increase of the T-reg-recruiting chemokine CCL22 expression in a progressive course of cervical dysplasia. J Cancer Res Clin Oncol 149, 6613–6623 (2023). https://doi.org/10.1007/s00432-023-04638-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04638-w