Abstract
Purpose
Tumor location and tumor node metastasis (TNM) stage guide treatment decisions in colorectal cancer (CRC) patients. However, patients with the same disease stage do not benefit equally from adjuvant therapy. Hence, there remains an urgent clinical need to identify prognostic and/or predictive biomarker(s) to personalize treatment decisions. In this exploratory study, we investigated whether our previously defined metabolic Warburg-subtypes can predict which CRC patients might derive survival benefit from adjuvant therapy.
Methods
Information regarding treatment (surgery only: n = 1451; adjuvant radiotherapy: n = 82; or adjuvant chemotherapy: n = 260) and Warburg-subtype (Warburg-low: n = 485, -moderate: n = 641, or –high: n = 667) was available for 1793 CRC patients from the Netherlands Cohort Study (NLCS). Kaplan–Meier curves and Cox regression models were used to investigate survival benefit from adjuvant therapy compared to surgery-only for the different Warburg-subtypes.
Results
Patients with Warburg-moderate CRC (HRCRC-specific 0.64; 95% CI 0.47–0.86, HRoverall 0.61; 95% CI 0.47–0.80), and possibly Warburg-high CRC (HRCRC-specific 0.86; 95% CI 0.65–1.14, HRoverall 0.82; 95% CI 0.64–1.05), had survival benefit from adjuvant therapy. No survival benefit was observed for patients with Warburg-low CRC (HRCRC-specific 1.07; 95% CI 0.76–1.52, HRoverall 0.95; 95% CI 0.70–1.30). There was a significant interaction between Warburg-subtype and adjuvant therapy for CRC-specific survival (p = 0.049) and overall survival (p = 0.035).
Conclusion
Our results suggest that Warburg-subtypes may predict survival benefit from adjuvant therapy in CRC patients. A survival benefit from adjuvant therapy was observed for patients with Warburg-moderate and possibly Warburg-high CRC, but not for patients with Warburg-low CRC. Future prospective studies are necessary to validate our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second-leading cause of cancer-related death worldwide, accounting for more than 900,000 deaths in 2020 (Rawla et al. 2019; Ferlay et al. 2020). Currently, tumor location and tumor node metastasis (TNM) stage guide treatment decisions in CRC patients (Kawakami et al. 2015; Roelands et al. 2017). However, patients with the same disease stage can have different survival and response to adjuvant therapy (Kawakami et al. 2015; Sinicrope et al. 2016, Roelands et al. 2017; Zhai et al. 2017; Ji et al. 2018). This may be due to heterogeneity in patient or tumor characteristics (Kawakami et al. 2015; Sinicrope et al. 2016; Roelands et al. 2017; Zhai et al. 2017; Ji et al. 2018).
Currently, there is only a limited number of biomarkers to identify CRC patients who are most likely to benefit from adjuvant therapy (Ji et al. 2018). Molecular classification of CRC may identify patient subgroups at high risk for recurrence and death, thereby facilitating the selection of patients for (personalized) therapy (Kawakami et al. 2015; Sinicrope et al. 2016). However, to date, only assessment of DNA mismatch repair (MMR) status and RAS and BRAF mutation status have been integrated into routine clinical practice to select patients for specific therapies (Fontana et al. 2019, Ten Hoorn et al. 2022). Hence, there remains an urgent clinical need to identify novel prognostic and/or predictive biomarker(s) to improve survival and quality of life in CRC patients (Ji et al. 2018, Ten Hoorn et al. 2022).
Metabolic reprogramming is one of the recognized hallmarks of cancer (Hanahan and Weinberg 2011). Otto Warburg first described in the 1920s, that cancer cells increase their glucose uptake and lactate secretion, even in the presence of oxygen (Warburg et al. 1927; Bensinger and Christofk 2012; Kato et al. 2018; Wolpaw and Dang 2018). This phenomenon of aerobic glycolysis, also known as the “Warburg-effect”, has since been observed in a variety of cancer types, including CRC (Sakashita et al. 2001; Potter et al. 2016).
We previously classified CRC as Warburg-low (i.e., low probability of the presence of the Warburg-effect), Warburg-moderate, or Warburg-high using a pathway-based sum score based on the expression levels of six glycolytic proteins, including transcriptional regulators, indicative of the Warburg-effect (LDHA, GLUT1, MCT4, PKM2, p53, and PTEN) (Jenniskens et al. 2021a; Offermans et al. 2021; Jenniskens et al. 2022). Our previous results, based on the total cohort of CRC patients, indicated that the Warburg-high subtype was associated with a poor survival in CRC patients, independent of known prognostic factors like TNM stage (Offermans et al. 2021).
Many studies have investigated the relationship between cellular metabolism and therapy resistance in CRC (Liu et al. 2021). The majority of studies suggested that the Warburg-effect promotes tumor characteristics that contribute to adjuvant therapy resistance (Morandi and Indraccolo 2017; Zhong and Zhou 2017; Zaal and Berkers 2018; Desbats et al. 2020; Kitazawa et al. 2020; Liu et al. 2021; Dong et al. 2022). However, most current evidence is based on in vitro cell culture studies, whereas—to the best of our knowledge—evidence from prospective cohort studies is lacking.
We hypothesized that patients with Warburg-high CRC will not derive a survival benefit from adjuvant chemo- or radiotherapy, whereas patients with Warburg-low CRC will derive survival benefit from adjuvant therapy. In this exploratory study, we therefore aimed to investigate whether our previously defined Warburg-subtypes can be used to predict survival benefit from adjuvant therapy in CRC patients.
Methods
Design and study population
The population-based series of colorectal cancer (CRC) patients in this study was derived from the prospective Netherlands Cohort Study (NLCS), which has been described in detail previously (van den Brandt et al. 1990a). Briefly, the NLCS was initiated in September 1986 and included 120,852 men and women, aged 55–69 years old, who completed a mailed, self-administered questionnaire on diet and other cancer risk factors at baseline (van den Brandt et al. 1990a). Participants agreed to participate in the study by completing and returning the questionnaire.
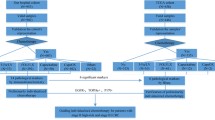
The entire prospective cohort was followed up for cancer incidence by annual record linkage with the Netherlands Cancer Registry and PALGA, the nationwide Dutch Pathology Registry (van den Brandt et al. 1990b; Casparie et al. 2007), covering 20.3 years of follow-up (September 17, 1986 until January 1, 2007). The completeness of cancer incidence follow-up was estimated to be > 96% (Goldbohm et al. 1994). After excluding patients who reported a history of cancer (excluding non-melanoma skin cancer) at baseline, 4597 incident CRC patients were available (Fig. 1).
The NLCS was approved by the institutional review boards of the TNO Quality of Life Research Institute (Zeist, the Netherlands) and Maastricht University (Maastricht, the Netherlands). Ethical approval for this study was obtained from the Medical Ethical Committee (METC) of Maastricht University Medical Center + .
Establishing Warburg-subtypes based on Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) tissue blocks from CRC resection specimens, excluding CRC patients who received neo-adjuvant chemotherapy (n = 10), were collected as part of the Rainbow-Tissue MicroArray (TMA) project (van den Brandt 2018). Details regarding TMA construction have been described previously (Offermans et al. 2021).
In total, 78 TMA blocks were constructed containing three 0.6 mm cores from tumor and three from normal epithelium of 2694 CRC patients (Fig. 1). Serial sections (5 µm) were subjected to immunohistochemistry (IHC) for Warburg-related proteins (LDHA, GLUT1, MCT4, PKM2, p53, PTEN) and mismatch-repair (MMR)-related proteins (MLH1, MSH2), as described previously (Jenniskens et al. 2021a, b; Offermans et al. 2021; Jenniskens et al. 2022).
Requiring at least one tumor core per patient, 2497 CRC patients passed quality control (Fig. 1). Multiple core-level IHC scores were combined into patient-level Warburg-subtypes as described previously (Jenniskens et al. 2021a, b; Offermans et al. 2021; Jenniskens et al. 2022). After excluding patients with missing IHC data, 2394 CRC patients were categorized as “Warburg-low” (n = 695, 29.0%), “Warburg-moderate” (n = 858, 35.8%) or “Warburg-high” (n = 841, 35.1%) subtype.
Clinical characteristics and follow-up
Follow-up for vital status of the CRC patients was carried out through linkage to the Central Bureau of Genealogy and the municipal population registries until December 31, 2012. Patients who were found to have CRC at autopsy (n = 5), patients with incomplete data regarding initial treatment (n = 21), patients who did not receive any treatment (no surgery, chemo- or radiotherapy; n = 8), patients who received another type of therapy (n = 7), or patients who received neo-adjuvant radiotherapy (n = 143) were excluded. Furthermore, patients with TNM stage I CRC (n = 422), who were mostly treated with surgery only (n = 412, 97.6%), were excluded from analyses to ensure that patients in the surgery only subgroup had similar clinical characteristics as patients in the adjuvant therapy subgroup. Hence, 1,793 CRC patients were available for analyses (Fig. 1).
Causes of death were retrieved from Statistics Netherlands. CRC-specific deaths included patients with an underlying cause attributed to malignant neoplasms of the colon, rectosigmoid junction, or rectum. Overall vital status was available for 1,792 (99.9%) patients and CRC-specific vital status for 1,765 (98.4%) patients.
Information about age at diagnosis, pTNM stage, tumor location, tumor differentiation grade, and primary adjuvant therapy (i.e., treatments included in the initial treatment plan drawn up after diagnosis) was retrieved from the cancer registry or PALGA histopathology reports. The cancer registry only registered information regarding the primary treatment that was performed.
Statistical analyses
Descriptive statistics were calculated for clinical characteristics, using mean (standard deviation) or median (range) for continuous data and frequencies (percentage) for categorical data. For categorical variables, differences across treatment subgroups (i.e., surgery only, surgery and adjuvant radiotherapy, surgery and adjuvant chemotherapy) were evaluated using chi-squared (χ2) tests. For continuous variables, the distributions across groups were evaluated using Kruskal–Wallis tests.
The primary outcomes were CRC-specific survival (time from CRC diagnosis to CRC-related death or end of follow-up) and overall survival (time from CRC diagnosis to death from any cause or end of follow-up). Survival analyses were restricted to 10 years of follow-up because of the limited number of events in the later period (CRC-specific deaths: n = 22; overall deaths: n = 175). Kaplan–Meier curves were estimated to examine survival benefit from adjuvant therapy for the different Warburg-subtypes (Warburg-low, Warburg-moderate, and Warburg-high). Differences between survival curves were investigated using Wilcoxon tests.
In addition, Cox proportional hazards regression was used to estimate Hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between adjuvant therapy and survival by Warburg-subtype. The proportional hazards assumption was tested using the scaled Schoenfeld residuals (Schoenfeld 1982), by evaluating -log–log transformed survival curves or by introducing time–covariate interactions into the models. HRs were adjusted for a set of a priori selected prognostic factors: age at diagnosis (years); sex (men, women); tumor location (colon, rectosigmoid, rectum); pTNM stage (II, III, IV, unknown); differentiation grade (well, moderate, poor/undifferentiated, unknown); and MMR deficiency (no, yes, unknown). Year of diagnosis and pTNM version were considered as potential confounders and were retained in the models if they altered HRs by more than 10% (Kamangar 2012; Alexander et al. 2015). A separate category (‘unknown’) was used for patients with unknown clinical information regarding pTNM stage or differentiation grade to enable inclusion of these patients in the Cox proportional hazards models.
Disease stage was based on the pTNM classification according to the edition valid at the time of surgery, resulting in the use of five different TNM editions (UICC TNM editions 3–6), as described previously (Offermans et al. 2021). However, the main TNM stage groupings (I/II/III/IV) remained essentially unchanged (Sobin et al. 2010).
Sensitivity analyses, excluding CRC patients with unknown clinical information regarding TNM stage and differentiation grade (n = 143), yielded similar results (data not shown).
All analyses were conducted in Stata Statistical Software: Release 16 (StataCorp., College Station, TX). Two-sided p values < 0.05 were considered significant.
Results
Clinical characteristics
Clinical characteristics of the 1793 included colorectal cancer (CRC) patients according to therapy are presented in Table 1. The large majority (n = 1451, 80.9%) of CRC patients from the prospective Netherlands Cohort Study (NLCS) were treated with surgery only, while 82 (4.6%) and 260 (14.5%) patients were treated with adjuvant radio- or chemotherapy, respectively. The use of adjuvant chemotherapy increased over time (from 1.3% in 1986–1988 to 13.4% in 2004–2006), whereas the administration of adjuvant radiotherapy decreased (from 10.5% in 1986–1988 to 0.0% in 2004–2006; p < 0.001).
CRC patients treated with adjuvant radio- or chemotherapy were younger compared to patients treated with surgery only (median age at diagnosis 69.0 years and 72.0 years versus 75.0 years, respectively; p < 0.001). Men were more frequently treated with adjuvant radio- or chemotherapy compared to women (5.4% and 16.4% of men versus 3.6% and 12.2% of women, respectively; p = 0.004). Patients with colon cancers were more often treated with surgery only compared to patients with rectosigmoid or rectal cancers (84.4% versus 75.8% and 61.0%, respectively; p < 0.001). Furthermore, patients with rectal cancers were more often treated with adjuvant radiotherapy compared to patients with rectosigmoid or colon cancers (28.8% versus 7.9% and 0.7%, respectively). Patients with pTNM stage III or IV CRC more often received adjuvant chemotherapy compared to patients with pTNM stage II CRC (27.3% and 25.8% versus 2.2%, respectively; p < 0.001). Patients who were treated with adjuvant radio- or chemotherapy were, in retrospect, more likely to have MMR proficient CRC (MMRproficient 5.1% and 15.3% versus MMRdeficient 0.9% and 9.4%, respectively; p = 0.002).
Warburg-subtypes and survival after adjuvant therapy
The median follow-up time since diagnosis was 3.72 years (range: 0.0027 to 25.49 years). Survival analyses were restricted to 10 years of follow-up, because of the limited number of events in the later period. During these first 10 years of follow-up, 1243 (69.3%) deaths were observed, of which 848 (68.2%) were CRC-related deaths.
Association between adjuvant therapy and survival according to Warburg-subtype
In patients with Warburg-low CRC, univariable Kaplan–Meier curves showed significant differences in CRC-specific survival (pCRC-specific = 0.047), but not overall survival (poverall = 0.394), between treatment groups (Figs. 2A, 3A). Patients with Warburg-low CRC treated with adjuvant (chemo)therapy had a significantly worse CRC-specific survival compared to patients with Warburg-low CRC treated with surgery only (HRadjuvant therapy 1.63; 95% CI 1.20–2.20 and HRadjuvant chemotherapy 1.75; 95% CI 1.25–2.45; Table 2). These associations with survival disappeared after adjustment for confounders in multivariable-adjusted analyses (HRadjuvant therapy 1.07; 95% CI 0.76–1.52 and HRadjuvant chemotherapy 1.03; 95% CI 0.70–1.51; Table 2).
Univariable Kaplan–Meier curves showing CRC-specific survival of colorectal cancer patients within the Netherlands Cohort Study (NLCS, 1986–2006) for A Total CRC, B Warburg-low CRC, C Warburg-moderate CRC, or D Warburg-high CRC, according to the treatment received (surgery only, surgery and adjuvant radiotherapy, surgery adjuvant chemotherapy). RT radiotherapy, CHT chemotherapy
Univariable Kaplan–Meier curves showing overall survival of colorectal cancer patients within the Netherlands Cohort Study (NLCS, 1986–2006) for A Total CRC, B Warburg-low CRC, C Warburg-moderate CRC, or D Warburg-high CRC, according to the treatment received (surgery only, surgery and adjuvant radiotherapy, surgery and adjuvant chemotherapy). RT radiotherapy, CHT chemotherapy
In patients with Warburg-moderate CRC, univariable Kaplan–Meier curves showed significant differences in overall survival (poverall = 0.041), but not CRC-specific survival (pCRC-specific = 0.397), between treatment groups (Figs. 2B, 3B). Patients with Warburg-moderate CRC treated with adjuvant (chemo)therapy had a better overall survival compared to patients with Warburg-moderate CRC treated with surgery only (HRadjuvant therapy 0.81; 95% CI 0.64–1.03 and HRadjuvant chemotherapy 0.77; 95% CI 0.58–1.02; Table 2). In multivariable-adjusted analyses, these inverse associations with survival became even stronger and reached statistical significance for both CRC-specific (HRadjuvant therapy 0.64; 95% CI 0.47–0.86 and HRadjuvant chemotherapy 0.53; 95% CI 0.38–0.75; Table 2) and overall survival (HRadjuvant therapy 0.61; 95% CI 0.47–0.80 and HRadjuvant chemotherapy 0.50; 95% CI 0.37–0.67; Table 2).
In patients with Warburg-high CRC, univariable Kaplan–Meier curves showed significant differences in CRC-specific survival (pCRC-specific = 0.019), but not overall survival (poverall = 0.288), between treatment groups (Figs. 2B, 3B). Patients with Warburg-high CRC treated with adjuvant (chemo)therapy had a significantly worse CRC-specific (HRadjuvant therapy 1.58; 95% CI 1.23–2.02, HRadjuvant chemotherapy 1.67; 95% CI 1.27–2.18) and overall survival (HRadjuvant therapy 1.31; 95% CI 1.05–1.62, HRadjuvant chemotherapy 1.31; 95% CI 1.03–1.67) compared to patients with Warburg-high CRC treated with surgery only (Table 2). In multivariable-adjusted analyses, these associations with survival changed direction but did not reach statistical significance (CRC-specific survival: HRadjuvant therapy 0.86; 95% 0.65–1.14; overall survival: HRadjuvant therapy 0.82; 95% CI 0.64–1.05; Table 2). However, the association between adjuvant chemotherapy and overall survival did reach statistical significance (HRadjuvant chemotherapy 0.75; 95% CI 0.57–0.98; Table 2).
The interaction between Warburg-subtype and adjuvant therapy as calculated in a multivariable-adjusted Cox proportional hazard model, adjusted for age at diagnosis, sex, tumor location, TNM stage, differentiation grade, MMR status and year of diagnosis was statistically significant for CRC-specific survival (p = 0.049) and overall survival (p = 0.035).
In stratified analyses according to disease stage (Supplementary Table S1), similar trends were observed for patients with pTNM stage III CRC. However, in patients with pTNM stage II CRC, no significant association between adjuvant therapy and survival was observed for any of the Warburg-subtypes. In contrast, in patients with pTNM stage IV CRC, a significantly better survival was observed for patients with Warburg-low or Warburg-moderate CRC receiving adjuvant (chemo)therapy compared to patients who received surgery only. In stratified analyses according to tumor location (Supplementary Table S2), a significantly better survival was observed for patients with Warburg-moderate or Warburg-high cancers located in the colon who received adjuvant (chemo)therapy compared to patients who received surgery only. Furthermore, a significant survival benefit was observed for patients with Warburg-moderate cancers located in the rectum who received adjuvant (radio)therapy.
Discussion
In this large, population-based series of colorectal cancer (CRC) patients, we investigated whether our previously defined immunohistochemistry (IHC)-based Warburg-subtypes can be used to predict survival benefit from adjuvant therapy. Our results indicate that Warburg-subtypes may predict treatment benefit in CRC patients. While in general patients with stage II–IV CRC who received adjuvant (chemo)therapy had a significantly favorable CRC-specific and overall survival compared to patients who received surgery only, this benefit was only observed in patients with Warburg-moderate CRC. Patients with Warburg-high CRC also seemed to benefit from adjuvant therapy, but associations did not reach statistical significance. In contrast, no benefit from adjuvant (chemo)therapy was found for patients with Warburg-low CRC.
Since the 1950s, 5-fluorouracil (5-FU)-based chemotherapy remains the main pharmacological treatment modality for patients with CRC (Van der Jeught et al. 2018). Although the administration of chemotherapy can improve the survival of cancer patients, chemotherapy resistance remains a major problem (Liu et al. 2021). In CRC, 5-FU-based chemotherapy remains ineffective in approximately 30% of patients (Kitazawa et al. 2020). Hence, there remains an urgent clinical need to identify novel prognostic and/or predictive biomarker(s) to improve survival and quality of life in CRC patients (Ji et al. 2018; Ten Hoorn et al. 2022).
To the best of our knowledge, we are the first to prospectively investigate whether Warburg-subtypes are associated with adjuvant (chemo)therapy resistance in a large population-based cohort of CRC patients. Nevertheless, many studies have investigated the relationship between cellular metabolism and therapy resistance in vitro (Liu et al. 2021). Moreover, one retrospective study has investigated the relation between expression patterns of proteins related to the Warburg-effect and response to therapy in patient tissue samples (Kitazawa et al. 2020). On the one hand, the majority of studies suggest that aerobic glycolysis promotes tumor characteristics that contribute to adjuvant therapy resistance (Morandi and Indraccolo 2017; Zhong and Zhou 2017; Zaal and Berkers 2018; Desbats et al. 2020; Kitazawa et al. 2020; Liu et al. 2021; Dong et al. 2022). On the other hand, there are studies that suggest that therapy resistance is accompanied by a metabolic shift from aerobic glycolysis toward oxidative phosphorylation (OXPHOS) (Denise et al. 2015; Vellinga et al. 2015; Taniguchi et al. 2016). Assuming that the Warburg-high subtype represents CRC that rely mainly on aerobic glycolysis to meet their metabolic demands, whereas the Warburg-low subtype represents a more oxidative metabolic phenotype (i.e., OXPHOS), our results are in contrast with those of the majority of previous studies which showed that aerobic glycolysis is associated with adjuvant therapy resistance (Liu et al. 2021; Dong et al. 2022; Zaal and Berkers 2018; Desbats et al. 2020; Zhong and Zhou 2017; Morandi and Indraccolo 2017).
Even though future studies are necessary to validate our results and to further investigate the biological mechanisms, the discrepancy in results might be explained by the fact that previous reports were mostly based on in vitro cell culture studies (Morandi and Indraccolo 2017) or were conducted retrospectively (Kitazawa et al. 2020). It has been reported that in vitro conditions differ drastically from the conditions found in vivo in the tumor microenvironment (Pampaloni et al. 2007; Vermeersch et al. 2014). Furthermore, it has been suggested that the effect of therapy might differ depending on the environment in which the cancer cells reside (Jo et al. 2018). For example, research suggests that cancer cells may be sensitive to chemotherapy in cell culture, but become resistant when transplanted into animal models (Trédan et al. 2007).
A potential explanation for the observation that patients with Warburg-low CRC had no survival benefit from adjuvant (chemo)therapy has been described by Vellinga et al. (2015). Normally, the amount of adenosine 5’-triphosphate (ATP) that is generated by aerobic glycolysis is sufficient to support tumor cell growth and basal DNA repair activity (Gottesman et al. 2002; Vellinga et al. 2015). However, when chemotherapy is administered, the cellular ATP demand in cancer cells increases significantly as many enzymes involved in DNA repair, drug efflux, and drug detoxification require ATP to function (Gottesman et al. 2002; Vellinga et al. 2015). As OXPHOS is the most efficient way to generate ATP (Vander Heiden et al. 2009), cancer cells may switch from aerobic glycolysis to OXPHOS at times of high ATP demand (Vellinga et al. 2015). In line with our results, this may suggest that patients with Warburg-low CRC (i.e., patients with cancers that rely mainly on oxidative metabolism) are more capable of repairing DNA damage and regulating drug metabolism compared to patients with Warburg-moderate and Warburg-high CRC (i.e., patients with cancers that rely mainly on aerobic glycolysis), rendering them more resistant to adjuvant therapy.
Our results suggest that the predictive value of Warburg-subtypes may be limited to TNM stage III CRC. In TNM stage II, no survival benefit from adjuvant (chemo)therapy was observed for any of the Warburg-subtypes, while in TNM stage IV, all CRC patients had survival benefit from adjuvant (chemo)therapy regardless of Warburg-subtype. As adjuvant chemotherapy is the standard of care for TNM stage III CRC (Kornmann et al. 2008), and chemotherapy resistance is still a major problem in clinical practice (Kitazawa et al. 2020; Liu et al. 2021), Warburg-subtypes may in future help to determine which stage III CRC patients will benefit most from adjuvant (chemo)therapy.
The main strengths of the present study include the use of a large population-based series of incident CRC patients, the prospective design, the nearly complete follow-up, and the availability of tumor material for a large number of CRC patients. Our study has some limitations. First, we did not have a validation cohort available to confirm the observed associations. Second, we did not have any detailed clinical information available regarding the dosage, duration or exact type of treatment. Third, we did not adjust for multiple testing which may have potentially resulted in chance findings. Fourth, in the Netherlands Cohort Study (NLCS), the large majority of CRC patients were treated with surgery only, resulting in a relatively small number of patients that were treated with adjuvant therapy, thereby limiting the power of our analyses. However, the limited amount of patients treated with adjuvant therapy was representative for this time period (1986–2006) (Van Steenbergen et al. 2010). Lastly, limitations with regard to Warburg-subtyping were described in detail previously (Offermans et al. 2021).
Conclusion
In conclusion, Warburg-subtypes may predict treatment benefit in CRC patients. Our results suggest that survival benefit from adjuvant (chemo)therapy in patients with CRC may depend on Warburg-subtype. Opposite to expectation, a survival benefit from adjuvant (chemo)therapy was observed for patients with Warburg-moderate and possibly also Warburg-high CRC, but not for patients with Warburg-low CRC.
All in all, our results highlight the importance of molecular classification of CRC based on Warburg-related proteins, in addition to TNM stage and tumor location, to identify subgroups of patients who are more likely to benefit from adjuvant (chemo)therapy. However, as this is an exploratory study, our results should be interpreted with caution and future prospective studies are necessary to validate our findings.
Data availability
The datasets generated and/or analysed during the current study are not publicly available because the informed consent does not allow for that.
Abbreviations
- 5-FU:
-
5-Fluorouracil
- ATP:
-
Adenosine 5'-triphosphate
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- FFPE:
-
Formalin-fixed paraffin-embedded
- HR:
-
Hazard ratio
- METC:
-
Medical Ethical Committee
- MMR:
-
Mismatch repair
- NLCS:
-
Netherlands Cohort Study
- OXPHOS:
-
Oxidative phosphorylation
- PALGA:
-
Dutch Pathology Registry
- TMA:
-
Tissue MicroArray
- TNM:
-
Tumor node metastasis
References
Alexander LK, Lopes B, Ricchetti-Masterson K, Yeatts KB (2015) Confounding bias, part II and effect measure modification. UNC CH Department of Epidemiology
Bensinger SJ, Christofk HR (2012) New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol 23(4):352–361
Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA (2007) Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 29(1):19–24
Denise C, Paoli P, Calvani M, Taddei ML, Giannoni E, Kopetz S, Kazmi SMA, Pia MM, Pettazzoni P, Sacco E (2015) 5-fluorouracil resistant colon cancer cells are addicted to OXPHOS to survive and enhance stem-like traits. Oncotarget 6(39):41706
Desbats MA, Giacomini I, Prayer-Galetti T, Montopoli M (2020) Metabolic plasticity in chemotherapy resistance. Front Oncol 10:281
Dong S, Liang S, Cheng Z, Zhang X, Luo L, Li L, Zhang W, Li S, Xu Q, Zhong M (2022) ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J Exp Clin Cancer Res 41(1):1–27
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2020) Global Cancer Observatory: Cancer Today. Available at https://gco.iarc.fr/today. Accessed 15 Feb 2021
Fontana E, Eason K, Cervantes A, Salazar R, Sadanandam A (2019) Context matters—consensus molecular subtypes of colorectal cancer as biomarkers for clinical trials. Ann Oncol 30(4):520–527
Goldbohm RA, van den Brandt PA, Dorant E (1994) Estimation of the coverage of municipalities by cancer registries and PALGA using hospital discharge data. Tijdschr Soc Gezondheidsz 72:80–84
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer 2(1):48–58
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Jenniskens JC, Offermans K, Samarska I, Fazzi GE, Simons CC, Smits KM, Schouten LJ, Weijenberg MP, van den Brandt PA, Grabsch HI (2021a) Validity and reproducibility of immunohistochemical scoring by trained non-pathologists on Tissue MicroArrays. Cancer Epidemiol Prev Biomarkers 30(10):1867–1874
Jenniskens JC, Offermans K, Simons CC, Samarska I, Fazzi GE, Smits KM, Schouten LJ, Weijenberg MP, Grabsch HI, van den Brandt PA (2021b) Energy balance-related factors and risk of colorectal cancer expressing different levels of proteins involved in the Warburg-effect. Cancer Epidemiol Prev Biomarkers 31(3):633–646
Jenniskens JC, Offermans K, Simons CC, Samarska I, Fazzi GE, Smits KM, Schouten LJ, Weijenberg MP, Grabsch HI, van den Brandt PA (2022) Energy balance-related factors in childhood and adolescence and risk of colorectal cancer expressing different levels of proteins involved in the Warburg-effect. Int J Cancer 150(11):1812–1824
Ji D, Zhan T, Li M, Yao Y, Jia J, Yi H, Qiao M, Xia J, Zhang Z, Ding H (2018) Enhancement of sensitivity to chemo/radiation therapy by using miR-15b against DCLK1 in colorectal cancer. Stem Cell Reports 11(6):1506–1522
Jo Y, Choi N, Kim K, Koo H-J, Choi J, Kim HN (2018) Chemoresistance of cancer cells: requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics 8(19):5259
Kamangar F (2012) Confounding variables in epidemiologic studies: basics and beyond. Arch Iran Med 15(8):508–516
Kato Y, Maeda T, Suzuki A, Baba Y (2018) Cancer metabolism: New insights into classic characteristics. Jpn Dent Sci Rev 54(1):8–21
Kawakami H, Zaanan A, Sinicrope FA (2015) Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol 16(7):1–15
Kitazawa M, Hatta T, Sasaki Y, Fukui K, Ogawa K, Fukuda E, Goshima N, Okita N, Yamada Y, Nakagama H (2020) Promotion of the Warburg effect is associated with poor benefit from adjuvant chemotherapy in colorectal cancer. Cancer Sci 111(2):658
Kornmann M, Formentini A, Ette C, Henne-Bruns D, Kron M, Sander S, Baumann W, Kreuser E-D, Staib L, Link K (2008) Prognostic factors influencing the survival of patients with colon cancer receiving adjuvant 5-FU treatment. Eur J Surg Oncol (EJSO) 34(12):1316–1321
Liu C, Jin Y, Fan Z (2021) The mechanism of Warburg effect-induced chemoresistance in cancer. Front Oncol 11:698023
Morandi A, Indraccolo S (2017) Linking metabolic reprogramming to therapy resistance in cancer. Biochimica Et Biophysica Acta (BBA)- Rev Cancer 1868(1):1–6
Offermans K, Jenniskens JC, Simons CC, Samarska I, Fazzi GE, Smits KM, Schouten LJ, Weijenberg MP, Grabsch HI, van den Brandt PA (2021) Expression of proteins associated with the Warburg-effect and survival in colorectal cancer. J Pathol: Clin Res 8(2):169–180
Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8(10):839–845
Potter M, Newport E, Morten KJ (2016) The Warburg effect: 80 years on. Biochem Soc Trans 44(5):1499–1505
Rawla P, Sunkara T, Barsouk A (2019) Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 14(2):89–103
Roelands J, Kuppen PJ, Vermeulen L, Maccalli C, Decock J, Wang E, Marincola FM, Bedognetti D, Hendrickx W (2017) Immunogenomic classification of colorectal cancer and therapeutic implications. Int J Mol Sci 18(10):2229
Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, Kuroda Y, Maeda S, Kasuga M (2001) Glut1 expression in T1 and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur J Cancer 37(2):204–209
Schoenfeld D (1982) Partial residuals for the proportional hazards regression model. Biometrika 69(1):239–241
Sinicrope FA, Okamoto K, Kasi PM, Kawakami H (2016) Molecular biomarkers in the personalized treatment of colorectal cancer. Clin Gastroenterol Hepatol 14(5):651–658
Sobin LH, Compton CA, Gospodarowicz M, Greene FL, Gunderson LL, Jessup JM, Wittekind C (2010) ‘Evidence-based medicine: the time has come to set standards for staging’. Is a radical overhaul really needed? J Pathol 221(4):361–362
Taniguchi K, Sakai M, Sugito N, Kuranaga Y, Kumazaki M, Shinohara H, Ueda H, Futamura M, Yoshida K, Uchiyama K (2016) PKM1 is involved in resistance to anti-cancer drugs. Biochem Biophys Res Commun 473(1):174–180
Ten Hoorn S, de Back TR, Sommeijer DW, Vermeulen L (2022) Clinical value of consensus molecular subtypes in colorectal cancer: a systematic review and meta-analysis. J Natl Cancer Inst 114(4):503–516
Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99(19):1441–1454
van den Brandt PA (2018) Molecular pathological epidemiology of lifestyle factors and colorectal and renal cell cancer risk. Maastricht Pathology 2018. 11th Joint Meeting of the British Division of the International Academy of Pathology and the Pathological Society of Great Britain & Ireland, 19–22 June 2018. J Pathol 246(Suppl 1):S9
van den Brandt PA, Goldbohm RA, van’t Veer P, Volovics A, Hermus RJ, Sturmans F (1990a) A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol 43(3):285–295
van den Brandt PA, Schouten LJ, Goldbohm RA, Dorant E, Hunen PM (1990b) Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int J Epidemiol 19(3):553–558
Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G (2018) Drug resistance and new therapies in colorectal cancer. World J Gastroenterol 24(34):3834–3848
Van Steenbergen L, Elferink M, Krijnen P, Lemmens V, Siesling S, Rutten H, Richel D, Karim-Kos H, Coebergh J (2010) Improved survival of colon cancer due to improved treatment and detection: a nationwide population-based study in The Netherlands 1989–2006. Ann Oncol 21(11):2206–2212
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Vellinga TT, Borovski T, de Boer VC, Fatrai S, van Schelven S, Trumpi K, Verheem A, Snoeren N, Emmink BL, Koster J (2015) SIRT1/PGC1α-dependent increase in oxidative phosphorylation supports chemotherapy resistance of colon cancer. Clin Cancer Res 21(12):2870–2879
Vermeersch KA, Wang L, McDonald JF, Styczynski MP (2014) Distinct metabolic responses of an ovarian cancer stem cell line. BMC Syst Biol 8(1):1–14
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8(6):519–530
Wolpaw AJ, Dang CV (2018) Exploiting metabolic vulnerabilities of cancer with precision and accuracy. Trends Cell Biol 28(3):201–212
Zaal EA, Berkers CR (2018) The influence of metabolism on drug response in cancer. Front Oncol 8:500
Zhai Z, Yu X, Yang B, Zhang Y, Zhang L, Li X, Sun H (2017) Colorectal cancer heterogeneity and targeted therapy: clinical implications, challenges and solutions for treatment resistance. Seminars in cell & developmental biology. Elsevier
Zhong J-T, Zhou S-H (2017) Warburg effect, hexokinase-II, and radioresistance of laryngeal carcinoma. Oncotarget 8(8):14133
Acknowledgements
The authors would like to thank the participants and staff of the Netherlands Cohort Study (NLCS), the Netherlands Cancer Registry, and the Dutch Pathology Registry. They are grateful to Ron Alofs and Harry van Montfort for data management and programming assistance; to Jaleesa van der Meer, Edith van den Boezem, and Peter Moerkerk for TMA construction; and to Jakob Kather (University Hospital Aachen, Germany) for scanning of TMA slides. The Rainbow-TMA consortium was financially supported by BBMRI-NL, a Research Infrastructure financed by the Dutch government (NWO 184.021.007, to P.A. van den Brandt), and Maastricht University Medical Center, University Medical Center Utrecht, and Radboud University Medical Centre, the Netherlands. The authors would like to thank all investigators from the Rainbow-TMA consortium project group [P.A. van den Brandt, A. zur Hausen, H. Grabsch, M. van Engeland, L.J. Schouten, J. Beckervordersandforth (Maastricht University Medical Center, Maastricht, Netherlands); P.H.M. Peeters, P.J. van Diest, H.B. Bueno de Mesquita (University Medical Center Utrecht, Utrecht, Netherlands); J. van Krieken, I. Nagtegaal, B. Siebers, B. Kiemeney (Radboud University Medical Center, Nijmegen, Netherlands); F.J. van Kemenade, C. Steegers, D. Boomsma, G.A. Meijer (VU University Medical Center, Amsterdam, Netherlands); F.J. van Kemenade, B. Stricker (Erasmus University Medical Center, Rotterdam, Netherlands); L. Overbeek, A. Gijsbers (PALGA, the Nationwide Histopathology and Cytopathology Data Network and Archive, Houten, Netherlands)] and collaborating pathologists [Amongst others: A. de Bruïne (VieCuri Medical Center, Venlo); J.C. Beckervordersandforth (Maastricht University Medical Center, Maastricht); J. van Krieken, I. Nagtegaal (Radboud University Medical Center, Nijmegen); W. Timens (University Medical Center Groningen, Groningen); F.J. van Kemenade (Erasmus University Medical Center, Rotterdam); M.C.H. Hogenes (Laboratory for Pathology OostNederland, Hengelo); P.J. van Diest (University Medical Center Utrecht, Utrecht); R.E. Kibbelaar (Pathology Friesland, Leeuwarden); A.F. Hamel (Stichting Samenwerkende Ziekenhuizen Oost-Groningen, Winschoten); A.T.M.G. Tiebosch (Martini Hospital, Groningen); C. Meijers (Reinier de Graaf Gasthuis/ S.S.D.Z., Delft); R. Natté (Haga Hospital Leyenburg, The Hague); G.A. Meijer (VU University Medical Center, Amsterdam); J.J.T.H. Roelofs (Academic Medical Center, Amsterdam); R.F. Hoedemaeker (Pathology Laboratory Pathan, Rotterdam); S. Sastrowijoto (Orbis Medical Center, Sittard); M. Nap (Atrium Medical Center, Heerlen); H.T. Shirango (Deventer Hospital, Deventer); H. Doornewaard (Gelre Hospital, Apeldoorn); J.E. Boers (Isala Hospital, Zwolle); J.C. van der Linden (Jeroen Bosch Hospital, Den Bosch); G. Burger (Symbiant Pathology Center, Alkmaar); R.W. Rouse (Meander Medical Center, Amersfoort); P.C. de Bruin (St. Antonius Hospital, Nieuwegein); P. Drillenburg (Onze Lieve Vrouwe Gasthuis, Amsterdam); C. van Krimpen (Kennemer Gasthuis, Haarlem); J.F. Graadt van Roggen (Diaconessenhuis, Leiden); S.A.J. Loyson (Bronovo Hospital, The Hague); J.D. Rupa (Laurentius Hospital, Roermond); H. Kliffen (Maasstad Hospital, Rotterdam); H.M. Hazelbag (Medical Center Haaglanden, The Hague); K. Schelfout (Stichting Pathologisch en Cytologisch Laboratorium West-Brabant, Bergen op Zoom); J. Stavast (Laboratorium Klinische Pathologie Centraal Brabant, Tilburg); I. van Lijnschoten (PAMM laboratory for Pathology and Medical Microbiology, Eindhoven); K. Duthoi (Amphia Hospital, Breda)].
Funding
This project was funded by The Dutch Cancer Society (KWF 11044 to P.A. van den Brandt). The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
PvdB, HG, CS, JJ, KO: conceptualization; PvdB: methodology; PvdB, LS: data acquisition; KO: formal analysis and investigation; PvdB, HG, CS, JJ, KO: writing—original draft preparation; PvdB, HG, CS, MW, LS, KS, IS, GF, JJ: writing—review and editing; PvdB, HG: funding acquisition; PvdB, HG: supervision.
Corresponding authors
Ethics declarations
Conflict of interest
HG has received honoraria from Astra Zeneca and BMS for scientific advisory board activities not related to the current study. The remaining authors have no conflicts of interest to declare.
Ethical approval
The NLCS was approved by institutional review boards from Maastricht University and the Netherlands Organization for Applied Scientific Research. Ethical approval was obtained from Medical Ethical Committee of Maastricht University Medical Center + . By completing and returning the questionnaire, participants agreed to participate in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Offermans, K., Jenniskens, J.C.A., Simons, C.C.J.M. et al. Association between adjuvant therapy and survival in colorectal cancer patients according to metabolic Warburg-subtypes. J Cancer Res Clin Oncol 149, 6271–6282 (2023). https://doi.org/10.1007/s00432-023-04581-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04581-w