Abstract
Purpose
Although RAS and PIK3CA mutations have been associated with resistance to anti-EGFR antibody in colorectal cancer or trastuzumab in breast cancer, their implications for trastuzumab resistance in HER2-positive advanced gastric cancer (AGC) remains unclear. We aimed to assess the relationship between trastuzumab efficacy and mutation status in the HER family signaling pathway.
Methods
This study retrospectively evaluated patients with HER2-positive AGC who received first-line trastuzumab-containing chemotherapy between March 2011 and November 2015. Multiplex genotyping, including KRAS, NRAS, PIK3CA, and BRAF, was then performed using the Luminex Assay, after which KRAS amplification was measured using quantitative real-time reverse transcription-polymerase chain reaction. Thereafter, the association between genetic alterations and clinical outcomes were evaluated.
Results
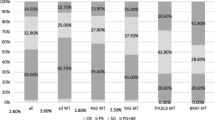
KRAS mutation (MT) was detected in 6 of 77 patients (7.8%), whereas KRAS amplification was found in 15 of 67 patients (22%). No mutations in NRAS, PIK3CA, or BRAF were identified. The KRAS MT group showed significantly worse response rates (16.7% vs. 66.2%, P = 0.016), progression-free survival [median, 4.8 vs. 11.6 months; hazard ratio (HR), 3.95; 95% CI, 1.60–9.76; P = 0.0029], and overall survival (11.5 vs. 23.6 months; HR, 3.80; 95% CI, 1.56–9.28; P = 0.033) compared to the KRAS wild-type group. KRAS amplification had no effect on clinical outcomes.
Conclusion
KRAS mutation was an independent prognostic factor for poor survival and might predict insufficient trastuzumab efficacy, whereas KRAS amplification showed no prognostic significance during trastuzumab treatment. Further investigations are warranted to confirm the predictive value of KRAS status in HER2-positive AGC.




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- AGC:
-
Advanced gastric cancer
- CI:
-
Confidence interval
- DCR:
-
Disease control rate
- FFPE:
-
Formalin-fixed paraffin-embedded
- FISH:
-
Fluorescent in situ hybridization
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- MT:
-
Mutation
- NLR:
-
Neutrophil-to-lymphocyte ratio
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RR:
-
Response rate
- RECIST:
-
Response evaluation criteria in solid tumors
- SD:
-
Standard distribution
- ULN:
-
Upper limit of normal
- WT:
-
Wild-type
References
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ et al (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634. https://doi.org/10.1200/JCO.2007.14.7116
Bai B, Shan L, Xie B, Huang X, Mao W, Wang X et al (2018) Mutations in KRAS codon 12 predict poor survival in Chinese patients with metastatic colorectal cancer. Oncol Lett 15:3161–3166. https://doi.org/10.3892/ol.2017.7709
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697. https://doi.org/10.1016/S0140-6736(10)61121-X
Barok M, Isola J, Palyi-Krekk Z, Nagy P, Juhasz I, Vereb G et al (2007) Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther 6:2065–2072. https://doi.org/10.1158/1535-7163.MCT-06-0766
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K et al (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12:395–402. https://doi.org/10.1016/j.ccr.2007.08.030
Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202–209. https://doi.org/10.1038/nature13480
Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ (2004) Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer–pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 22:2395–2403. https://doi.org/10.1200/JCO.2004.08.154
Cizkova M, Dujaric ME, Lehmann-Che J, Scott V, Tembo O, Asselain B et al (2013) Outcome impact of PIK3CA mutations in HER2-positive breast cancer patients treated with trastuzumab. Br J Cancer 108:1807–1809. https://doi.org/10.1038/bjc.2013.164
Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M et al (2019) Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol 5:343–350. https://doi.org/10.1001/jamaoncol.2018.5080
Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA (2011) Systematic review: anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med 154:37–49. https://doi.org/10.7326/0003-4819-154-1-201101040-00006
Deguchi Y, Okabe H, Oshima N, Hisamori S, Minamiguchi S, Muto M et al (2017) PTEN loss is associated with a poor response to trastuzumab in HER2-overexpressing gastroesophageal adenocarcinoma. Gastric Cancer 20:416–427. https://doi.org/10.1007/s10120-016-0627-z
Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB et al (2012) A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61:673–684. https://doi.org/10.1136/gutjnl-2011-301839
Duraes C, Almeida GM, Seruca R, Oliveira C, Carneiro F (2014) Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch 464:367–378. https://doi.org/10.1007/s00428-013-1533-y
Favazza LA, Parseghian CM, Kaya C, Nikiforova MN, Roy S, Wald AI et al (2020) KRAS amplification in metastatic colon cancer is associated with a history of inflammatory bowel disease and may confer resistance to anti-EGFR therapy. Mod Pathol 33:1832–1843. https://doi.org/10.1038/s41379-020-0560-x
Gravalos C, Jimeno A (2008) HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 19:1523–1529. https://doi.org/10.1093/annonc/mdn169
Hanker AB, Brown BP, Meiler J, Marin A, Jayanthan HS, Ye D et al (2021) Co-occurring gain-of-function mutations in HER2 and HER3 modulate HER2/HER3 activation, oncogenesis, and HER2 inhibitor sensitivity. Cancer Cell 39:1099–1114. https://doi.org/10.1016/j.ccell.2021.06.001
Hewitt LC, Hutchins GG, Melotte V, Saito Y, Grabsch HI (2015) KRAS, BRAF and gastric cancer. Transl Gastrointest Cancer. https://doi.org/10.3978/j.issn.2224-4778.2015.09.08
Hudis CA (2007) Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med 357:39–51. https://doi.org/10.1056/NEJMra043186
Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI et al (2018) HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554:189–194. https://doi.org/10.1038/nature25475
Janjigian YY, Sanchez-Vega F, Jonsson P, Chatila WK, Hechtman JF, Ku GY et al (2018) Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 8:49–58. https://doi.org/10.1158/2159-8290.CD-17-0787
Jensen JD, Knoop A, Laenkholm AV, Grauslund M, Jensen MB, Santoni-Rugiu E et al (2012) PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome in HER2-positive early-stage breast cancer patients treated with adjuvant chemotherapy and trastuzumab. Ann Oncol 23:2034–2042. https://doi.org/10.1093/annonc/mdr546
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765. https://doi.org/10.1056/NEJMoa0804385
Kim JW, Lee HS, Nam KH, Ahn S, Kim JW, Ahn SH et al (2017) PIK3CA mutations are associated with increased tumor aggressiveness and Akt activation in gastric cancer. Oncotarget 8:90948–90958. https://doi.org/10.18632/oncotarget.18770
Klapper LN, Waterman H, Sela M, Yarden Y (2000) Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res 60:3384–3338
Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF et al (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992–3995. https://doi.org/10.1158/0008-5472.CAN-06-0191
Lordick F, Luber B, Lorenzen S, Hegewisch-Becker S, Folprecht G, Woll E et al (2010) Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer 102:500–505. https://doi.org/10.1038/sj.bjc.6605521
Makiyama A, Sukawa Y, Kashiwada T, Kawada J, Hosokawa A, Horie Y et al (2020) Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT Study). J Clin Oncol 38:1919–1927. https://doi.org/10.1200/JCO.19.03077
Matsusaka S, Kobunai T, Yamamoto N, Chin K, Ogura M, Tanaka G et al (2016) Prognostic impact of KRAS mutant type and MET amplification in metastatic and recurrent gastric cancer patients treated with first-line S-1 plus cisplatin chemotherapy. Genes Cancer 7:27–35. https://doi.org/10.18632/genesandcancer.96
Moehler M, Mueller A, Trarbach T, Lordick F, Seufferlein T, Kubicka S et al (2011) Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 22:1358–1366. https://doi.org/10.1093/annonc/mdq591
Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K et al (2019) Neutrophil-to-lymphocyte ratio as a prognostic indicator in patients with unresectable gastric cancer. Anticancer Res 39:2583–2589. https://doi.org/10.21873/anticanres.13381
Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K (2021) Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol 18:473–487. https://doi.org/10.1038/s41571-021-00492-2
Patra S, Young V, Llewellyn L, Senapati JN, Mathew J (2017) BRAF, KRAS and PIK3CA mutation and sensitivity to trastuzumab in breast cancer cell line model. Asian Pac J Cancer Prev 18:2209–2213. https://doi.org/10.22034/APJCP.2017.18.8.2209
Pietrantonio F, Fuca G, Morano F, Gloghini A, Corso S, Aprile G et al (2018) Biomarkers of primary resistance to trastuzumab in HER2-positive metastatic gastric cancer patients: the AMNESIA case-control study. Clin Cancer Res 24:1082–1089. https://doi.org/10.1158/1078-0432.CCR-17-2781
Polom K, Marrelli D, Roviello G, Pascale V, Voglino C, Vindigni C et al (2018) PIK3CA mutation in gastric cancer and the role of microsatellite instability status in mutations of exons 9 and 20 of the PIK3CA gene. Adv Clin Exp Med 27:963–969. https://doi.org/10.17219/acem/70795
Rehkaemper J, Korenkov M, Quaas A, Rueschoff J, Pamuk A, Zander T et al (2020) Amplification of KRAS and its heterogeneity in non-Asian gastric adenocarcinomas. BMC Cancer 20:587. https://doi.org/10.1186/s12885-020-06996-x
Ruschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G et al (2010) HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 457:299–307. https://doi.org/10.1007/s00428-010-0952-2
Sartore-Bianchi A, Pietrantonio F, Lonardi S, Mussolin B, Rua F, Fenocchio E et al (2021) Phase II study of anti-EGFR rechallenge therapy with panitumumab driven by circulating tumor DNA molecular selection in metastatic colorectal cancer: the CHRONOS trial. J Clin Oncol 39:3506. https://doi.org/10.1200/JCO.2021.39.15_suppl.3506
Shimozaki K, Nakayama I, Takahari D, Kamiimabeppu D, Osumi H, Wakatsuki T et al (2021) A novel clinical prognostic index for patients with advanced gastric cancer: possible contribution to the continuum of care. ESMO Open 6:100234. https://doi.org/10.1016/j.esmoop.2021.100234
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D et al (2020) Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 382:2419–2430. https://doi.org/10.1056/NEJMoa2004413
Shitara K, Bang Y, Iwasa S, Sugimoto N, Ryu M, Sakai D et al (2021) O-14 Exploratory biomarker analysis of trastuzumab deruxtecan in DESTINY-Gastric01, a randomized, phase 2, multicenter, open-label study in patients with HER2-positive or -low advanced gastric or gastroesophageal junction adenocarcinoma. Ann Oncol 32:S224. https://doi.org/10.1016/j.annonc.2021.05.018
Shojaei S, Gardaneh M, Rahimi Shamabadi A (2012) Target points in trastuzumab resistance. Int J Breast Cancer 2012:761917. https://doi.org/10.1155/2012/761917
Sunakawa Y, Nakamura M, Ishizaki M, Kataoka M, Satake H, Kitazono M et al (2020) RAS mutations in circulating tumor DNA and clinical outcomes of rechallenge treatment with anti-EGFR antibodies in patients with metastatic colorectal cancer. JCO Precis Oncol 4:898–911. https://doi.org/10.1200/po.20.00109
Takahari D, Mizusawa J, Koizumi W, Hyodo I, Boku N (2017) Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric Cancer 20:757–763. https://doi.org/10.1007/s10120-017-0702-0
Takahari D, Chin K, Ishizuka N, Takashima A, Minashi K, Kadowaki S et al (2019) Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naive, HER2-positive advanced gastric cancer. Gastric Cancer 22:1238–1246. https://doi.org/10.1007/s10120-019-00973-5
Tokunaga R, Imamura Y, Nakamura K, Ishimoto T, Nakagawa S, Miyake K et al (2016) Fibroblast growth factor receptor 2 expression, but not its genetic amplification, is associated with tumor growth and worse survival in esophagogastric junction adenocarcinoma. Oncotarget 7:19748–19761. https://doi.org/10.18632/oncotarget.7782
Valtorta E, Misale S, Sartore-Bianchi A, Nagtegaal ID, Paraf F, Lauricella C et al (2013) KRAS gene amplification in colorectal cancer and impact on response to EGFR-targeted therapy. Int J Cancer 133:1259–1265. https://doi.org/10.1002/ijc.28106
van Grieken NC, Aoyama T, Chambers PA, Bottomley D, Ward LC, Inam I et al (2013) KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br J Cancer 108:1495–1501. https://doi.org/10.1038/bjc.2013.109
von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE et al (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol 27:1999–2006. https://doi.org/10.1200/JCO.2008.19.6618
Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C et al (2013) Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 14:481–489. https://doi.org/10.1016/S1470-2045(13)70096-2
Wang DS, Liu ZX, Lu YX, Bao H, Wu X, Zeng ZL et al (2019) Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 68:1152–1161. https://doi.org/10.1136/gutjnl-2018-316522
Wong GS, Zhou J, Liu JB, Wu Z, Xu X, Li T et al (2018) Targeting wild-type KRAS-amplified gastroesophageal cancer through combined MEK and SHP2 inhibition. Nat Med 24:968–977. https://doi.org/10.1038/s41591-018-0022-x
Acknowledgements
The authors thank the patients, as well as their families and caregivers, who participated in this study.
Funding
This work was partly supported by grant from the Setsuro Fujii Memorial and the Osaka Foundation for the Promotion of Fundamental Medical Research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by KS and ES. The first draft of the manuscript was written by KS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
E. Shinozaki has received honoraria from Taiho, Merck Serono, Takeda, Chugai, Yakult, Ono, Bayer, and Lilly. All other authors declare no conflicts of interest.
Ethics approval
All procedures were performed in accordance with the Helsinki Declaration of 1964 and its later versions.
Consent to participate
Informed consent was obtained from all patients as a comprehensive requirement of research studies by the Japanese Foundation for Cancer Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shimozaki, K., Shinozaki, E., Yamamoto, N. et al. KRAS mutation as a predictor of insufficient trastuzumab efficacy and poor prognosis in HER2-positive advanced gastric cancer. J Cancer Res Clin Oncol 149, 1273–1283 (2023). https://doi.org/10.1007/s00432-022-03966-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03966-7
Keywords
Profiles
- Akira Ooki View author profile