Abstract
Purpose
Abscess or fistula of the anal region is an uncommon presentation of malignancy. Under the assumption of a benign condition, diagnostics is often delayed, resulting in advanced tumour stages at first diagnosis. Due to the case rarity, treatment guidelines for cancers of anorectal region masquerading as abscess or fistula are missing.
Methods
We analysed all patients presenting with an abscess or fistula of the anal region in our department between January 2004 and August 2020. The malignancies were included to our study to acquire data on clinical presentation, treatment and outcome. Furthermore, a systematic review to present adenocarcinomas and squamous cell carcinomas associated to an abscess or fistula was performed.
Results
0.5% of the patients treated for an abscess or fistula of the anal region met the selection criteria. Mean time from the onset of symptoms to diagnosis of malignancy was 100 days. Histology revealed adenocarcinoma and squamous cell carcinoma each in two patients. All patients had locally advanced tumours without distant metastases, in two cases with regional lymph-node metastases. Neoadjuvant chemoradiation was applied in two patients. All patients underwent abdomino-perineal resection of the rectum. The overall outcome reveals a recurrence-free survival of 4.5 and 3 years for two patients. Further two patients died within 5 months after the primary resection.
Conclusion
Advanced carcinomas of the anorectal region may masquerade as abscess or fistula, cause diagnostic problems and delay oncologic treatment. However, even in these very advanced situations, surgical therapy with curative intent should be attempted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections of the anal, perianal, perineal, sacral or gluteal region, such as abscesses and fistulas, are a common occurrence in surgical practice. The incidence of perianal abscesses is estimated around 15–20 per 100,000 inhabitants (Adamo et al. 2016). Sporadic fistulas occur in approximately one-third of the patients with perianal abscesses; furthermore, they are observed in 5–40% of patients with Crohn’s disease (Gold et al. 2018).
In contrast, malignant tumours of the same region are rare and represent approximately 1.5% of gastrointestinal malignancies (Salati and Al Kadi 2012). However, malignant tumours of the anal region can masquerade as inflammatory changes of the skin or fistulas, often rendering timely diagnosis (DS) extremely difficult (Klas et al. 1999; Leong et al. 2019; Ohta et al. 2013). Therefore, these patients usually present with advanced tumour stages (Ohta et al. 2013; Kapiteijn et al. 2001). For these rare cases, there is no consensus regarding diagnostic and treatment strategies (Yang et al. 2009). Previous reports mostly consist of case reports, in which multimodal treatment and extensive surgery are recommended, despite differences in the histological findings (Leong et al. 2019; Benjelloun et al. 2012; Gaertner et al. 2008; Pai et al. 2015).
Perianal malignancies arising from abscesses or fistulas can be adenocarcinomas (ADC) or squamous cell carcinomas (SCC). Of these two entities, adenocarcinomas are more common (2.9–10%) (Chandramohan et al. 2010; Marti et al. 2001). The adenocarcinomas can be divided into 3–6 further subgroups, of which mucinous adenocarcinomas (MAC) are reported most frequently in the context of fistulas and abscesses (Yang et al. 2009; Marti et al. 2001; Diaz-Vico et al. 2019; Maternini et al. 2018; Venclauskas et al. 2009). Mucinous adenocarcinomas derive from epithelial tissue and according to WHO classification, the diagnosis requires the secretion of extracellular mucin more than 50% of the tumour volume (Xie et al. 2018; Bosman 2010). The impact of mucinous histology on prognosis in colorectal adenocarcinomas however is yet unclear, with some studies revealing shorter overall survival compared to non-MACs (Xie et al. 2018; Soliman et al. 2016) and others, who did not find any adverse prognostic effect (Xie et al. 2018; Hogan 2013). A population-based study using the data from the Surveillance, Epidemiology and End Results (SEER) program reported a difference in survival outcomes of mucinous adenocarcinomas depending on primary tumour site; therefore, tailored treatment should be applied (Xie et al. 2018).
The aim of this paper is to report our experiences with patients who were treated for an abscess or fistula of the perianal skin and finally diagnosed with cancer. Clinical presentation, multidisciplinary management, surgical procedures and outcome are presented. Furthermore, we performed a systematic review to compare our data with previous reports, focusing on non-MACs and SCCs diagnosed in abscesses or fistulas in the perianal region.
Methods
Retrospective data analysis
All patients who presented in our institution between January 2004 and August 2020 with an abscess or fistula of the anal, perianal, perineal, sacral or gluteal region and eventually underwent surgery for a malignant tumour were included in this survey. The data were extracted from our electronic medical records (SAP® i.s.h.med®) using following ICD-10 codes: K61.0–K61.4, L02.3, K60.0–K60.5. External patient data were archived in our records for patients primarily treated in peripheral hospitals and referred to us due to the case complexity. Basic demographic data, information on clinical presentation, time to diagnosis, treatment and outcome were included in the analysis. Collection of the patient’s data and the study were approved by the local ethics committee of the University Hospital Jena under the registration number 2020–2001. Mean was used for continuous variables. Statistical analysis was performed with SPSS software (IBM Company, version 23, IBM Corporation, Armonk, NY, USA).
Systematic review
A systematic search was performed using the database Medline® Library. The search was last actualized in September 2020. Inclusion criteria were full paper publications in peer-reviewed journals reporting original works, case reports or systematic reviews.
The search string for ADC was “adenocarcinoma”[Title] AND “abscess”[Title], “adenocarcinoma”[Title] AND “fistula”[Title] and “adenocarcinoma”[Title] AND “chronic infection”[Title]. For SCC, a similar string was used (“squamous cell carcinoma”[Title] AND “abscess”[Title], “squamous cell carcinoma”[Title] AND “fistula”[Title] and “squamous cell carcinoma”[Title] AND “chronic infection”[Title]). A filter was set for language (English), and mucinous adenocarcinomas, hidradenitis suppurativa as well as chronic inflammatory diseases such as Crohn’s disease were excluded.
According to the title/abstract screening, all publications meeting the inclusion criteria were retrieved as full text. The data extracted included the study authors, the publication year, the number of cases, the treatment, and the outcome. The review was performed in accordance with the PRISMA statement (Fig. 1, Moher et al. 2009).
Assessment of eligible articles according to PRISMA 2009 Flow Diagram (Moher et al. 2009)
Results
Retrospective data analysis (Table 1)
Demographic data
Between January 2004 and August 2020, 807 patients have been treated for anal, perianal, perineal, sacral or gluteal abscess or fistula in our department. The majority of these patients were male (m) (72%). Four patients of this cohort with an abscess of the gluteal region were later diagnosed with a malignancy. The clinical and demographical features are depicted in Table 1. The mean age at the time of tumour diagnosis was 54.5 years (y); two patients were male and two female (f).
Clinical presentation and time from symptoms onset to therapy (Fig. 2)
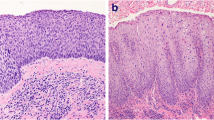
Exemplary presentation of case 1 (Table 1). a Clinical presentation at time of consultation in our department, b histological specimen after APR with extensive resection of skin, gluteus muscle and S3-5, c wound surface after resection prior to free latissimus muscle flap
All patients primarily presented in external hospitals after a mean time of 48 days following the onset of the symptoms. Three of the four patients reported gluteal or anal pain as the leading symptom; the remaining patient sought medical advice for a non-healing wound at the sacrum. Consequently, the primary treatment at the external hospitals consisted of drainage/excision of the presumed abscess in two of the four patients. The other two patients were referred to our department shortly after clinical examination under the suspicion of a malignant tumour (1 and 18 days after the first medical consultation). The mean time of referral to our department following the first medical consultation for all patients was 55 days. This results in a mean time from the onset of symptoms to first diagnosis of malignancy as much as 100 days. With two patients having received neoadjuvant chemoradiation, the mean time from the first symptoms to the first oncological surgery was 167 days.
Preoperative staging
Preoperative diagnostics included a computer tomography (CT) of the chest, the abdomen and the pelvis, a magnetic resonance imaging (MRI) of the pelvis as well as a rectoscopy/colonoscopy in all patients (Figs. 3, 4, 5, 6).
Patient c1; first MRI of the pelvis, 8 days prior to first histology of malignancy: Signal enhancement around the femoral head on the right as well as the right sacrum. Pathologically enlarged lymph nodes bilaterally in the groin area. Fistulas in the subcutaneous tissue. Inflammation in the gluteal muscles right > left. Fistula-like fluid accumulations along the inflammatory areas, minor fluid accumulations presacral and dorsal to the rectum
Patient c2; first MRI of the pelvis, 1 day prior to first histology of malignancy: Large, ulcerated, space occupying lesion median/paramedian on both sides gluteally from sacral vertebrae 3 to the pelvic floor, approximately 14 cm × 12 cm × 6 cm in size. Irregular configuration at the margins. Extension of the lesion cutaneously, subcutaneously and muscularly into the adjacent parts of the gluteus maximus, minimus and medius muscles as well as the piriformis muscle and the levator ani muscle. Further extension to the sacrum and the coccyx, which appears destructed. Perifocal edema. Lymph node with contrast medium enrichment at left gluteus. Pathologically enlarged iliac and inguinal lymph nodes bilaterally
Patient c4; first CT of the pelvis, 1 day prior to first histology of malignancy: Suspicion of a large, abscess forming inflammatory lesion pararectally with air entrapments and therefore suspicious of a connection to the rectum. No evidence of fistula. Diffuse inflammatory swelling of the gluteal muscles and the subcutaneous tissue at right gluteus. Pathologically enlarged lymph nodes in the ischiorectal fossa and presacral
Surgical treatment
All patients underwent abdomino-perineal resection of the rectum including partial resection of the sacrum in three patients. For both patients with SCC (c1 and c2), extensive resection of gluteus maximus muscles and skin was required to achieve free resection margins. The Nigro protocol could not be applied, because the clinical findings were consistent with squamous cell carcinoma of the perianal skin with deep infiltration rather than classic anal carcinoma. Furthermore, taking into account the extension of the tumour, the irradiation field would have been too large. Secondary reconstruction was performed using free latissimus-dorsi muscle flaps. In a third patient (c4), simultaneous reconstruction could be achieved with a vertical rectus abdominis myocutaneous flap (VRAM-flap).
Histological findings
Histology revealed ADC in both female patients and SCC in the two male patients. All patients had locally advanced tumours (pT 3 or 4) without distant metastases at the time of diagnosis, in two cases with regional lymph-node metastases (pN3 and pN1b).
Outcome
The mean length of hospital stay (LOS) was 59 days due to a prolonged wound healing necessitating recurrent operative wound debridements in two patients (c2 and 3, Clavien–Dindo IIIb).
One male patient with associated hemophilia A died 3 months after the tumour operation due to necrosis of the latissimus flap requiring multiple operative interventions (c1). Furthermore, a pleural carcinosis leading to respiratory failure was diagnosed in the postoperative course. Distant metastases (metachronous liver metastases) occurred in a patient 2 months after the resection of the primary tumour; she died due to liver and respiratory failure caused by an influenza infection 28 days following the partial liver resection (c4). The remaining two patients are tumour-free after a follow-up of 54 and 36 months after the oncological operation.
Systematic review
The search disclosed a total of 302 articles containing the keywords ADC or SCC and abscess or fistula (Fig. 1). 11 studies covering the period from 1982 to 2019 met our selection criteria; 4 of them reporting ADC and 7 SCC.
Discussion
Initially, 302 records containing our previously mentioned keywords were found with database searching. 291 articles were excluded after abstract screening for not matching the inclusion criteria. The most frequent histology found in the tumours were MACs (Leong et al. 2019; Gaertner et al. 2008; Gomes et al. 2014; Rakoto-Ratsimba et al. 2006). Due to their largely unknown histopathological behaviour (Xie et al. 2018) and to enable a comparability with our patients, MACs were excluded from analyses.
Adenocarcinoma associated with abscess or fistula, n = 4 (Table 2)
Except Benjelloun et al. (2012), all authors presenting ADC associated with abscess or fistula, reported both MACs and non-MACs. However, as mentioned, only the cases with non-MACs were included to our analyses. The patients were all male and had a history of recurrent perianal infection (0.5–40 years) with the majority suffering from fistula.
The first case described by Benjelloun et al. (2012) had a history of anal fistula of 10 years; 3 months after the last fistulectomy, an ADC was diagnosed. In all remaining case reports, the tumour diagnosis was at first clinical treatment (Leong et al. 2019; Benjelloun et al. 2012; Hongo et al. 2013; Tan et al. 1989).
Tan et al. (1989) performed a barium enema study that showed an irregular narrowed area of the sigmoid colon; further staging modalities are not reported. Benjelloun et al. (2012) and Leong et al. (2019) staged the patients with preoperative CT scan, MRI and endoscopy. Hongo et al. (2013) mostly performed a contrast enhanced CT scan and only added MRI in a few cases to assess the local extent of disease. Additionally, both cases reported by Benjelloun et al. (2012) received endoanal ultrasound.
The majority of the patients initially received local surgical therapies for infect control. Tan et al. (1989) immediately performed sigmoid colectomy with excision of upper rectum and fistulectomy. At the second step, a wide perineal resection with excision of the anorectal stump was done to have tumour-free margins. An inferior gluteal thigh flap was used for reconstruction of the defect. The patient reported by Leong et al. (2019) was treated with chemoradiation due to metastatic disease. All other patients received APR with (Benjelloun et al. 2012) or without (Hongo et al. 2013) local excision of perianal mass.
The outcome of the patients described in the cited cases varies. Tan et al. (1989) did not indicate the outcome. The patient reported by Leong et al. ( 2019) died due to the development of metastases 5 months after first diagnosis upon completion of CR for a pT4 N0 M0 ADC. Distant recurrence-free survival and local recurrence-free survival ranged from 11 to 52 months in the cases presented by Benjelloun et al. (2012) and Hongo et al. (2013). The maximum survival was 104 months (c4, Hongo et al. 2013) with death due to local recurrence. However, the exact time of recurrence is unclear.
Squamous cell carcinoma associated with abscess or fistula, n = 7 (Table 3)
The authors reported each 1 case of SCC associated with abscess or fistula (Chandramohan et al. 2010; Jamieson and Goode 1982; Seya et al. 2007; Moore et al. 2016; Creta et al. 2017; Garg et al. 2018; Mizusawa et al. 2019). In the majority of the cases, the patients were male (5/7), only 1 was female, and in 1 case, the sex was not reported.
4/7 patients had a history of recurrent infection for more than 20 years prior to the diagnosis of malignancy. The infections, either acute or chronic, have been in the anal, perineal or gluteal area. The patient presented by Jamieson et al. (1982) had a 20 years history of pilonidal sinus and the female patient reported by Seya et al. (2007) suffered from recurrent perianal abscesses with fistulas since 32 years. In one patient, the abscess was located in the gluteal region (Chandramohan et al. 2010). In the remaining 4 case reports, the infection was found in the perineal region followed by the delayed diagnosis of a urethral tumour (3 cases) (Moore et al. 2016; Garg et al. 2018; Mizusawa et al. 2019) or a carcinoma of unknown primary origin (CUP) (Creta et al. 2017) (delay of 2–18 months).
The staging examinations were not performed in a standardized way. For instance, Jamieson et al. (1982) performed an isotopic bone and a CT scan only after the third recurrence of the tumour; Seya et al. (2007), Chandramohan et al. (2010) and Creta et al. (2017) carried out endoscopic examination prior to the operation. Furthermore, Chandramohan et al. (2010), Moore et al. (2016), Creta et al. (2017), Garg et al. (2018) and Mizusawa et al. (2019) completed the staging with a cross-sectional imaging.
All patients were treated with minor local surgery at first presentation. In the case described by Jamieson et al. (1982), the first two recurrences were also treated by local resection. However, after the third recurrence, hemi-corporectomy was offered which was refused by the patient. For the patient reported by Seya et al. (2007), an APR with lymph-node dissection was performed. No further surgery was done for the patient in Chandramohan et al.s (2010) case report. Due to the delayed diagnosis of malignancy in the patients with urethral tumour and CUP, an extended resection was necessary (Moore et al. 2016; Creta et al. 2017; Garg et al. 2018; Mizusawa et al. 2019). However, only two patients were eligible for surgery (Moore et al. 2016; Mizusawa et al. 2019), one was in a poor general condition and therefore not suitable for en bloc tumour resection (Creta et al. 2017) and a further one refused an extended resection (Garg et al. 2018).
The outcome of all patients reported can be considered as rather poor. For two patients, the outcome was not mentioned (Chandramohan et al. 2010; Garg et al. 2018), and one patient was lost to follow up after 3 months (Creta et al. 2017). Three patients died after 17 (Mizusawa et al. 2019), 18 (Jamieson and Goode 1982) and 24 (Moore et al. 2016) months, respectively. The patient in Seya et al. (2007) report showed a recurrence-free survival of the SCC in the anal region for 10 years. However, 8 years after the first tumour diagnosis, an urothelial carcinoma of the urinary bladder was found, and the patient died 2 years after the resection of the second tumour due to disseminated disease.
The systematic review reveals that consistent treatment concepts are missing. Individual decisions are taken concerning diagnostics and therapy, according to the tumour extension. This might be due to the case rarity. In the reported cases for both entities, there was a history of recurrent abscess or fistula for 0.5–40 years. This gives rise to the assumption, that the tumour derived due to the chronic infection (malignant transformation). It remains unclear, if the tumour diagnosis in these cases was delayed, because a long history of disease with frequent recurrence (Leong et al. 2019; Benjelloun et al. 2012; Chandramohan et al. 2010; Jamieson and Goode 1982; Seya et al. 2007; Moore et al. 2016) can often lead to an underestimation of the severeness. However, it is well known that the long-term risk of anal cancer is significantly increased in patients with inflammatory anal lesions (Nordenvall et al. 2006). Since Virchow’s hypothesis in 1863, namely that lymphoreticular infiltrate reflected the origin of cancer at sites of chronic inflammation (Virchow 1863), several types of cancer have been associated with infections (Balkwill and Mantovani 2001).
The prevalence of perianal abscess in men is higher (Adamo et al. 2016). Also, the incidence rate of invasive anal carcinoma in the United States is reported to be higher in men (Benson et al. 2018). The gender distribution in our systematic review depicts the trend that the occurrence of ADC and SCC seems to be more common in male patients. In our retrospective study, 2 of the tumour patients were male and 2 female.
In general, the malignancy appears to arise at a later age: the mean age at the time of diagnosis in the literature reviewed was 64.7 years (exact age was missing in one patient) and 54.5 years in our data set.
The case numbers in our department (n = 807 in 16 years) confirm the relatively high incidence of abscesses or fistulas in the anal region (Adamo et al. 2016) requiring minor surgical interventions. Furthermore, with only 0.5% of malignancy within our data set, our experience confirms that this condition is rather rare (Salati and Al Kadi 2012; Kline et al. 1964).
However, following the analysis of our data set, we report an important new finding. Malignancies can be associated with abscesses of the anal, perianal, perineal, sacral or gluteal region even if the history of disease is rather short compared to those described in the literature. The lesions often appear benign, and therefore, histological examination is frequently not performed at the time of initial diagnosis of an abscess (Moore et al. 2016; Garg et al. 2018), or false-negative biopsies occur (Leong et al. 2019). This considerably impedes the timely diagnosis, leads to advanced tumour stages and renders the therapy extremely difficult.
We can assume that both the patient and the surgeon are involved in the cause of late diagnosis. In our cohort, the patients presented after 48 days following the onset of their symptoms. After approximately twice the time, 100 days, the malignancy was proven. Due to a missing consensus for the treatment of those patients, individual therapy concepts have to be designed (Yang et al. 2009).
All of our patients received an extensive oncological surgery. 2 of them (c1 and c3) had neoadjuvant therapy, 1 (c4) adjuvant therapy and 1 (c2) had neither neoadjuvant nor adjuvant therapy. With this treatment concept, 2 of our 4 patients present a long-term survival with DRFS/LRFS of 4.5 (c2) and 3 (c3) years. Compared to the data in the literature review, this is a favourable outcome.
The significance of neoadjuvant or adjuvant chemoradiation is yet unclear. In case of ADCs, it seems reasonable to adhere to the guidelines for colorectal carcinomas and to apply neoadjuvant chemoradiation in the generally advanced carcinomas. For SCC, however, the situation is less clear. Both our patients reported here had very extensive involvement of the gluteal and sacral skin, unlike anal carcinoma. Even with partial response, the extent of surgery is likely not to be reduced, which was our reason to withhold neoadjuvant treatment in the second patient with SCC.
Conclusion
Despite the rarity of malignancies associated with abscesses and fistulas of the anal, perianal, perineal, sacral or gluteal region, histologic sample biopsies from the wound ground and/or the fistula canal should be assessed on a routine manner to prevent late diagnosis of malignancy. Especially, in cases of non-healing wounds and persistent pain, medical professionals should be suspicious. We would like to point out that, even in advanced tumour stages and regardless of the tumour entity, multimodal treatment concepts with an extensive surgery may lead to a promising outcome.
Ideally, a consensus guideline should be established for these cases to standardize treatment options and improve survival. However, due to the heterogeneity of the disease and its infrequent occurrence, this is not realistic. Treatment plans therefore should be discussed in multidisciplinary tumour boards and especially take the local resectability/need for downsizing into consideration.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Adamo K, Sandblom G, Brannstrom F, Strigard K (2016) Prevalence and recurrence rate of perianal abscess—a population-based study, Sweden 1997–2009. Int J Colorectal Dis 31(3):669–673. https://doi.org/10.1007/s00384-015-2500-7
Anal cancer incidence rates increased in antiretroviral era. Rates increased for men and women (2006) AIDS Alert 21 (3):22–23
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545. https://doi.org/10.1016/S0140-6736(00)04046-0
Benjelloun E, Aitalalim S, Chbani L, Mellouki I, Mazaz K, Aittaleb K (2012) Rectosigmoid adenocarcinoma revealed by metastatic anal fistula. The visible part of the iceberg: a report of two cases with literature review. World J Surg Oncol. https://doi.org/10.1186/1477-7819-10-209
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedmanass DA (2018) Anal carcinoma, version 22018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 16(7):852–871. https://doi.org/10.6004/jnccn.2018.0060
Bosman FT (2010) WHO classification of tumours of the digestive system, 4th edn. International Agency for Research on Cancer, Lyon
Chandramohan K, Mathew AP, Muralee M, Anila KR, Ramachandran K, Ahamed I (2010) Squamous cell carcinoma arising from long-standing perianal fistula. Int Wound J 7(6):515–518. https://doi.org/10.1111/j.1742-481X.2010.00724.x
Creta M, Mirone V, Di Meo S, Buonopane R, Longo N, Fusco F, Forte NR, Imperatore V (2017) A rare case of male pelvic squamous cell carcinoma of unknown primary origin presenting as perineal abscess and urethral stenosis. Arch Ital Urol Androl 89(2):154–155. https://doi.org/10.4081/aiua.2017.2.154
Diaz-Vico T, Fernandez-Martinez D, Garcia-Gutierrez C, Suarez-Sanchez A, Cifrian-Canales I, Mendoza-Pacas GE, Sanchez-Farpon H, Truan-Alonso N (2019) Mucinous adenocarcinoma arising from chronic perianal fistula-a multidisciplinary approach. J Gastrointest Oncol 10(3):589–596. https://doi.org/10.21037/jgo.2019.01.11
Gaertner WB, Hagerman GF, Finne CO, Alavi K, Jessurun J, Rothenberger DA, Madoff RD (2008) Fistula-associated anal adenocarcinoma: good results with aggressive therapy. Dis Colon Rectum 51(7):1061–1067. https://doi.org/10.1007/s10350-008-9294-4
Garg G, Mehdi S, Bansal N, Sankhwar S (2018) Squamous cell carcinoma of male urethra presenting as urethrocutaneous fistula. BMJ Case Rep. https://doi.org/10.1136/bcr-2018-227447
Gold SL, Cohen-Mekelburg S, Schneider Y, Steinlauf A (2018) Perianal fistulas in patients with Crohn’s disease, part 1: current medical management. Gastroenterol Hepatol (NY) 14(8):470–481
Gomes RM, Kumar RK, Desouza A, Saklani A (2014) Implantation metastasis from adenocarcinoma of the sigmoid colon into a perianal fistula: a case report. Ann Gastroenterol 27(3):276–279
Hogan JS, Burke JP, Waldron D (2013) Association of mucin production and survival in colon cancer. J Clin Oncol. https://doi.org/10.1200/jco.2013.31.4_suppl.512
Hongo K, Kazama S, Sunami E, Kitayama J, Watanabe T (2013) Perianal adenocarcinoma associated with anal fistula: a report of 11 cases in a single institution focusing on treatment and literature review. Hepatogastroenterology 60(124):720–726
Jamieson NV, Goode TB (1982) Squamous cell carcinoma arising in a pilonidal sinus presenting with the formation of an abscess. Postgrad Med J 58(685):720–721. https://doi.org/10.1136/pgmj.58.685.720
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ, Dutch Colorectal Cancer G (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345(9):638–646. https://doi.org/10.1056/NEJMoa010580
Klas JV, Rothenberger DA, Wong WD, Madoff RD (1999) Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer 85(8):1686–1693. https://doi.org/10.1002/(sici)1097-0142(19990415)85:8%3c1686::aid-cncr7%3e3.0.co;2-7
Kline RJ, Spencer RJ, Harrison EG Jr (1964) Carcinoma associated with fistula-in-ano. Arch Surg 89:989–994. https://doi.org/10.1001/archsurg.1964.01320060057011
Leong FQ, Chan DKH, Tan KK (2019) Anal adenocarcinoma can masquerade as chronic anal fistula in Asians. Ann Coloproctol 35(1):47–49. https://doi.org/10.3393/ac.2018.03.15
Marti L, Nussbaumer P, Breitbach T, Hollinger A (2001) Perianal mucinous adenocarcinoma. A further reason for histological study of anal fistula or anorectal abscess. Chirurg 72(5):573–577. https://doi.org/10.1007/s001040170137
Maternini M, Guttadauro A, Ripamonti L, Chiarelli M, Gabrielli F (2018) Malignant transformation of a chronic anorectal fistula. Ann Ital Chir 7:S2239253X18029109
Mizusawa H, Hara H, Mimura Y, Kato H (2019) Primary male urethral squamous cell carcinoma presenting with a genital abscess. IJU Case Rep 2(4):225–228. https://doi.org/10.1002/iju5.12090
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Moore SJ, Rashidipour O, Moore RB (2016) Primary metastatic squamous cell carcinoma of the male urethra presenting with scrotal abscess and subsequent development of fournier’s gangrene. Clin Med Insights Case Rep 9:83–86. https://doi.org/10.4137/CCRep.S40420
Nordenvall C, Nyren O, Ye W (2006) Elevated anal squamous cell carcinoma risk associated with benign inflammatory anal lesions. Gut 55(5):703–707. https://doi.org/10.1136/gut.2005.070201
Ohta R, Sekikawa K, Goto M, Narita K, Takahashi Y, Ikeda H, Oneyama M, Hirata Y, Nakayama M, Shimoda Y, Sato S (2013) A case of perianal mucinous adenocarcinoma arising from an anorectal fistula successfully resected after preoperative radiotherapy. Case Rep Gastroenterol 7(2):219–223. https://doi.org/10.1159/000351830
Pai VD, Jatal S, Engineer R, Ostwal V, Saklani AP (2015) Multidisciplinary management of colorectal adenocarcinoma associated with anal fistula: an Indian series. Colorectal Dis 17(11):O240-246. https://doi.org/10.1111/codi.13100
Rakoto-Ratsimba HN, Rakototiana AF, Rakotosamimanana J, Ranaivozanany A (2006) Anal adenocarcinoma revealed by a fistula-in-ano. Report of a case. Ann Chir 131(9):564–566. https://doi.org/10.1016/j.anchir.2006.03.019
Salati SA, Al Kadi A (2012) Anal cancer—a review. Int J Health Sci (qassim) 6(2):206–230. https://doi.org/10.12816/0006000
Seya T, Tanaka N, Shinji S, Yokoi K, Oguro T, Oaki Y, Ishiwata T, Naito Z, Tajiri T (2007) Squamous cell carcinoma arising from recurrent anal fistula. J Nippon Med School 74(4):319–324. https://doi.org/10.1272/jnms.74.319
Soliman BA, Amira G, Hamza H, Abbas H (2016) Mucinous colorectal carcinoma to predict poor outcome in young patients. J Clin Oncol. https://doi.org/10.1200/JCO.2016.34.15_suppl.e15076
Tan YS, Nambiar R, Sim CS (1989) Adenocarcinoma associated with chronic anal fistula. Ann Acad Med Singap 18(6):717–720
Venclauskas L, Saladzinskas Z, Tamelis A, Pranys D, Pavalkis D (2009) Mucinous adenocarcinoma arising in an anorectal fistula. Medicina (kaunas) 45(4):286–290
Virchow R (1863) Cellular pathology as based upon physiological and pathological histology. J. B. Lippincott, Philadelphia
Xie GD, Liu YR, Jiang YZ, Shao ZM (2018) Epidemiology and survival outcomes of mucinous adenocarcinomas: A SEER population-based study. Sci Rep 8(1):6117. https://doi.org/10.1038/s41598-018-24540-7
Yang BL, Shao WJ, Sun GD, Chen YQ, Huang JC (2009) Perianal mucinous adenocarcinoma arising from chronic anorectal fistulae: a review from single institution. Int J Colorectal Dis 24(9):1001–1006. https://doi.org/10.1007/s00384-009-0657-7
Funding
Open Access funding enabled and organized by Projekt DEAL. The author Aysun Tekbaș is funded by the Interdisciplinary Center for Clinical Research of the University of Jena, Germany.
Author information
Authors and Affiliations
Contributions
Study conception and design: AT, HM, US, and SS. Acquisition of data: AT, HM, and SS. Selection of papers for review: AT and SS. Analysis and interpretation of data: AT, HM, US, and SS. Drafting of manuscript: AT. Critical revision of manuscript: HM, US, and SS.
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare that there are no conflicts of interest.
Research including human participants/ethics approval
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. The study was registered by the local ethics committee under the registration number 2020–2001.
Consent to participate
Not applicable for retrospective data analyses.
Consent for publication
Not applicable for retrospective data analyses.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tekbaş, A., Mothes, H., Settmacher, U. et al. Non-mucinous adenocarcinomas and squamous cell carcinomas of the anal region masquerading as abscess or fistula: a retrospective analysis and systematic review of literature. J Cancer Res Clin Oncol 148, 1509–1522 (2022). https://doi.org/10.1007/s00432-021-03747-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03747-8