Abstract
Purpose
Our previous work identified leucine zipper transcription factor-like 1 (LZTFL1) as a novel tumor suppressor gene, with its expression correlated with survival outcome in gastric cancer (GC) patients. This study focuses on the role of LZTFL1 in GC aggression and metastasis as well as its underlying molecular mechanisms.
Method
LZTFL1 immunohistochemical (IHC) staining on 311 paired normal/cancer tissue arrays were used to reconfirm the clinical significance of LZTFL1 expression. Transwell chamber assays were used to determine migration and invasive ability of GC cells. Gelatin zymography was employed to investigate the matrix metalloproteinases (MMPs) activity in tumor cells. Co-immunoprecipitation and Duolink in situ proximity ligation assay were used to analyze the interaction between LZTFL1 and β-catenin and the cellular localization of the interaction.
Result
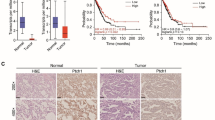
IHC results indicated that patients with high LZTFL1 expression had a longer overall survival time (58 months, 95 % CI 28–128 months) than patients with low LZTFL1 expression (27 months, 95 % CI 23–35 months; p < 0.01). The expression level of LZTFL1 is associated with the degree of cell differentiation. LZTFL1 is necessary and sufficient to inhibit the expression of molecular markers associated with epithelial–mesenchymal transition (EMT) and cellular phenotypes associated with tumor cell EMT including the migration, invasion, and the expression and activities of MMPs of tumor cells. LZTFL1 binds β-catenin in the cytoplasm of the cell and inhibited its nuclear translocation.
Conclusion
LZTFL1 suppresses GC cell EMT by inhibiting β-catenin nuclear translocation. Re-expression of LZTFL1 in GC cells may be a potential therapeutic means to prevent GC metastasis.






Similar content being viewed by others
References
Aigner K, Dampier B, Descovich L et al (2007) The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 26:6979–6988
Batlle E, Sancho E, Franci C et al (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89
Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M et al (2009) Recent patterns in gastric cancer: a global overview. Int J Cancer 125:666–673
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG et al (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Chan AO, Wong BC, Lan HY, Loke SL, Chan WK, Hui WM et al (2003) Deregulation of E-cadherin-catenin complex in precancerous lesions of gastric adenocarcinoma. J Gastroenterol Hepatol 18:534–539
Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A (2003) Autoregulation of E-cadherin expression by cadherin–cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol 163:847–857
Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P et al (1989) The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene 18:2883–2891
Di Croce L, Pelicci PG (2003) Tumour-associated hypermethylation: silencing E-cadherin expression enhances invasion and metastasis. Eur J Cancer 39:413–414
Floor S, van Staveren WC, Larsimont D, Dumont JE, Maenhaut C (2001) Cancer cells in epithelial-to-mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations. Oncogene 30:4609–4621
Gheldof A, Berx G (2013) Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci 116:317–336
Gomes LR, Terra LF, Sogayar MC, Labriola L (2011) Epithelial-mesenchymal transition: implications in cancer progression and metastasis. Curr Pharm Biotechnol 12:1881–1890
Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W (2010) HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol 32(1–2):57–65
Hajra KM, Chen DY, Fearon ER (2002) The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res 62:1613–1618
Hatsell S, Rowlands T, Hiremath M, Cowin P (2003) Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8(2):145–158
Hendriksen J, Jansen M, Brown CM, van der Velde H, van Ham M, Galjart N et al (2008) Plasma membrane recruitment of dephosphorylated beta-catenin upon activation of the Wnt pathway. J Cell Sci 121:1793–1802
Hesson LB, Cooper WN, Latif F (2007) Evaluation of the 3p21.3 tumor-suppressor gene cluster. Oncogene 26:7283–7301
Howe LR, Watanabe O, Leonard J, Brown AM (2003) Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res 63:1906–1913
Ieni A, Barresi V, Giuffrè G, Caruso RA, Lanzafame S, Villari L et al (2013) HER2 status in advanced gastric carcinoma: a retrospective multicentric analysis from Sicily. Oncol Lett 6:1591–1594
Ji L, Minna JD, Roth JA (2005) 3p21.3 tumor suppressor cluster: prospects for translational applications. Future Oncol 1:79–92
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67
Kiss H, Kedra D, Kiss C, Kost-Alimova M, Yang Y, Klein G et al (2001) The LZTFL1 gene is a part of a transcriptional map covering 250 kb within the common eliminated region 1 (C3CER1) in 3p21.3. Genomics 73:10–19
Lin CY, Tsai PH, Kandaswami CC, Lee PP, Huang CJ, Hwang JJ et al (2011) Matrix metalloproteinase-9 cooperates with transcription factor Snail to induce epithelial-mesenchymal transition. Cancer Sci 102:815–827
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN et al (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Marion V, Stutzmann F, Gérard M, De Melo C, Schaefer E, Claussmann A et al (2012) Exome sequencing identifies mutations in LZTFL1, a BBSome and smoothened trafficking regulator, in a family with Bardet–Biedl syndrome with situs inversus and insertional polydactyly. J Med Genet 49:317–321
Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW et al (1993) E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res 53:1690–1695
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B et al (1997) Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275:1787–1790
Murai T, Yamada S, Fuchs BC, Fujii T, Nakayama G, Sugimoto H et al (2014) Epithelial-to-mesenchymal transition predicts prognosis in clinical gastric cancer. J Surg Oncol 109:684–689
Niehrs C (2012) The complex world of WNT receptor signaling. Nat Rev Mol Cell Biol 13(12):767–779
Nisticò P, Bissell MJ, Radisky DC (2012) Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol 4(2):pii: a011908
Ohtsu A, Yoshida S, Saijo N (2006) Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol 24:2188–2196
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA (2008) Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 68:3645–3654
Orlichenko LS, Radisky DC (2008) Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis 25:593–600
Orsulic S, Huber O, Aberle H, Arnold S, Kemler R (1999) E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci 112:1237–1245
Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y et al (2010) Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 303:1729–1737
Park WS, Oh RR, Park JY, Lee SH, Shin MS, Kim YS et al (1999) Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res 59:4257–4260
Peleteiro B, Bastos A, Ferro A, Lunet N (2014) Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci. doi:10.1007/s10620-014-3063-0
Przybylo JA, Radisky DC (2007) Matrix metalloproteinase-induced epithelial-mesenchymal transition: tumor progression at Snail’s pace. Int J Biochem Cell Biol 39:1082–1088
Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER (1999) Frequent nuclear/cytoplasmic localization of beta-catenin without exon 3 mutations in malignant melanoma. Am J Pathol 154:325–329
Rosenbluh J, Wang X, Hahn WC (2014) Genomic insights into WNT/β-catenin signaling. Trends Pharmacol Sci 35(2):103–109
Rowlands TM, Pechenkina IV, Hatsell S, Cowin P (2004) Beta-catenin and cyclin D1: connecting development to breast cancer. Cell Cycle 3:145–148
Sasaki Y, Morimoto I, Kusano M, Hosokawa M, Itoh F, Yanagihara K, Imai K et al (2001) Mutational analysis of the beta-catenin gene in gastric carcinomas. Tumor Biol 22:123–130
Schmalhofer O, Brabletz S, Brabletz T (2009) E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev 28:151–166
Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV et al (2011) A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet 7:e1002358
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Vinson C, Acharya A, Taparowsky EJ (2006) Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim Biophys Acta 1759(1–2):4–12
Wagner AD, Grothe W, Behl S, Kleber G, Grothey A, Haerting J et al (2005) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev (2):CD004064. doi:10.1002/14651858.CD004064.pub3
Wang Q, Sun ZX, Allgayer H, Yang HS (2010) Downregulation of E-cadherin is an essential event in activating beta-catenin/Tcf-dependent transcription and expression of its target genes in Pdcd4 knockdown cells. Oncogene 29:128–138
Wei Q, Zhou W, Wang W, Gao B, Wang L, Cao J, Liu ZP (2010) Tumor-suppressive functions of leucine zipper transcription factor-like 1. Cancer Res 70:2942–2950
Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH (2001) Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer 5:108–113
Wu HW, Qin CY, Huang JL et al (2014) Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer. Asian Pac J Trop Med 7(4):257–261
Yokoyama K, Kamata N, Fujimoto R, Tsutsumi S, Tomonari M, Taki M et al (2003) Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol 22:891–898
Yoong J, Michael M, Leong T (2011) Targeted therapies for gastric cancer: current status. Drugs 71:1367–1384
Acknowledgments
This study was supported by National Natural Science Foundation of China to Q.W. (Grant No. 81101581) and National Institute of Health (NIHRO1HL10947) and Cancer Prevention and Research Institute of Texas (CPRIT-RP120717) to Z.P.L.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Linbo Wang and Jufeng Guo have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Guo, J., Wang, Q. et al. LZTFL1 suppresses gastric cancer cell migration and invasion through regulating nuclear translocation of β-catenin. J Cancer Res Clin Oncol 140, 1997–2008 (2014). https://doi.org/10.1007/s00432-014-1753-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1753-9