Abstract
Purpose
While macrophage migration inhibitory factor (MIF) has been extensively studied in the context of inflammation and inflammatory disorders, less work has been devoted to its involvement in cancer, notably in neoplastic progression. In a previous study, we have found evidence that MIF plays a role in head and neck squamous cell carcinomas (HNSCC). The current investigations were undertaken in order to estimate the importance of the MIF receptor, CD74 in the progression of HNSCC.
Methods and results
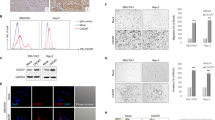
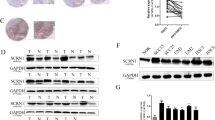
In a cohort of 46 cases of oral cavity carcinomas, immunohistochemical staining revealed an increase in CD74 expression during progression from benign lesions to carcinoma. As shown by cell culture experiments using squamous carcinoma cell line (SCCVII) transduced with anti-CD74 shRNA, the amount of cell-produced VEGF was lower in SCCVII CD74KD cell line compared with control SCCVII CD74sc cell line, suggesting that CD74 could be implicated in angiogenesis in vivo. Furthermore, knockdown of CD74 decreased proliferation of SCCVII cells in vitro. The migration of SCCVII cells, as well as the cell secretion of matrix metallopeptidase 9, was also negatively affected by CD74 knockdown. These observations in vitro were confirmed in an orthotopic mouse model of SCC where tumors produced by SCCVII CD74KD cell inoculation were found to grow more slowly than tumors generated by SCCVII CD74sc cells.
Conclusion
The clinical observations and experimental data reported here suggest that CD74, as well as MIF, plays a pivotal role in HNSCC progression.







Similar content being viewed by others
References
Cheng RJ, Deng WG, Niu CB, Li YY, Fu Y (2011) Expression of macrophage migration inhibitory factor and CD74 in cervical squamous cell carcinoma. Int J Gynecol Cancer 21:1004–1012
Choi JW, Kim Y, Lee JH, Kim YS (2013) CD74 expression is increased in high-grade, invasive urothelial carcinoma of the bladder. Int J Urol 20:251–255
Cludts S, Decaestecker C, Johnson B et al (2010) Increased expression of macrophage migration inhibitory factor during progression to hypopharyngeal squamous cell carcinoma. Anticancer Res 30:3313–3319
França CM, Batista AC, Borra RC et al (2013) Macrophage migration inhibitory factor and oral cancer. J Oral Pathol Med 42:368–373
Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, Balkwill FR (2007) Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther 6:1993–2002
Han J, Kioi M, Chu WS, Kasperbauer JL, Strome SE, Puri RK (2009) Identification of potential therapeutic targets in human head & neck squamous cell carcinoma. Head Neck Oncol 1:27
He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HH (2006) Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut 55:797–802
Hyams VJ, Batsakis JG, Michaels L (1988) Tumors of the upper respiratory tract and ear. In: Atlas of tumor pathology. Armed Forces Institute of Pathology, Washington, DC, pp 123–126
Journe F, Chaboteaux C, Dumon JC, Leclercq G, Laurent G, Body JJ (2004) Steroid-free medium discloses oestrogenic effects of the bisphosphonate clodronate on breast cancer cells. Br J Cancer 91:1703–1710
Jung H, Seong HA, Ha H (2008) Critical role of cysteine residue 81 of macrophage migration inhibitory factor (MIF) in MIF-induced inhibition of p53 activity. J Biol Chem 283:20383–20396
Khurana D, Martin EA, Kasperbauer JL et al (2001) Characterization of a spontaneously arising murine squamous cell carcinoma (SCCVII) as a prerequisite for head and neck cancer immunotherapy. Head Neck 23:899–906
Kindt N, Lechien J, Decaestecker C et al (2012) Expression of macrophage migration-inhibitory factor is correlated with progression in oral cavity carcinomas. Anticancer Res 32:4499–4505
Kindt N, Preillon J, Kaltner H et al (2013a) Macrophage migration inhibitory factor in head and neck squamous cell carcinoma: clinical and experimental studies. J Cancer Res Clin Oncol 139:727–737
Kindt N, Laurent G, Nonclercq D et al (2013b) Pharmacological inhibition of macrophage migration inhibitory factor interferes with the proliferation and invasiveness of squamous carcinoma cells. Int J Oncol 43:185–193
Krockenberger M, Engel JB, Kolb J et al (2010) Macrophage migration inhibitory factor expression in cervical cancer. J Cancer Res Clin Oncol 136:651–657
Leng L, Bucala R (2006) Insight into the biology of macrophage migration inhibitory factor (MIF) revealed by the cloning of its cell surface receptor. Cell Res 16:162–168
Leng L, Metz CN, Fang Y et al (2003) MIF signal transduction initiated by binding to CD74. J Exp Med 197:1467–1476
Lue H, Kapurniotu A, Fingerle-Rowson G et al (2006) Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal 18:688–703
Lue H, Thiele M, Franz J et al (2007) Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene 26:5046–5059
McClelland M, Zhao L, Carskadon S, Arenberg D (2009) Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol 174:638–646
Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL (2006) Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol 177:8730–8739
Molinolo AA, Amornphimoltham P, Squarize CH et al (2009) Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol 45:324–334
Nemajerova A, Moll UM, Petrenko O, Fingerle-Rowson G (2007) Macrophage migration inhibitory factor coordinates DNA damage response with the proteasomal control of the cell cycle. Cell Cycle 6:1030–1034
Oda S, Oda T, Nishi K et al (2008) Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS One 3:e2215
Pries R, Wollenberg B (2006) Cytokines in head and neck cancer. Cytokine Growth Factor Rev 17:141–146
Wang F, Arun P, Friedman J, Chen Z, Van Waes C (2009) Current and potential inflammation targeted therapies in head and neck cancer. Curr Opin Pharmacol 9:389–395
Wittekind C, Greene FL, Hutter RVP (2004) TNM atlas, 5th edn. UICC Springer, Berlin, pp 20–32
Acknowledgments
NK is the recipient of a fellowship from the “Fondation Rose and Jean Hoguet” and of a grant (“Televie”) from the National Fund for Scientific Research. GL is Senior Research Associate of the National Fund for Scientific Research (Belgium).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guy Laurent and Sven Saussez have contributed equally to this work and should be regarded as joint last authors.
Rights and permissions
About this article
Cite this article
Kindt, N., Lechien, J.R., Nonclercq, D. et al. Involvement of CD74 in head and neck squamous cell carcinomas. J Cancer Res Clin Oncol 140, 937–947 (2014). https://doi.org/10.1007/s00432-014-1648-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1648-9