Abstract
Objective
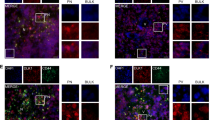
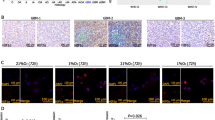
Wnt signalling pathways regulate proliferation, motility and survival in a variety of human cell types. Dickkopf 1 (DKK1) gene codes for a secreted Wnt inhibitory factor. It functions as tumour suppressor gene in breast cancer and as a pro-apoptotic factor in glioma cells. In this study, we aimed to demonstrate whether the different expression of DKK1 in human glioma-derived cells is dependent on microenvironmental factors like hypoxia and regulated by the intercellular crosstalk with bone-marrow-derived mesenchymal stem cells (bmMSCs).
Methods
Glioma cell line U87-MG, three cell lines from human glioblastoma grade IV (glioma-derived mesenchymal stem cells) and three bmMSCs were selected for the experiment. The expression of DKK1 in cell lines under normoxic/hypoxic environment or co-culture condition was measured using real-time PCR and enzyme-linked immunoadsorbent assay. The effect of DKK1 on cell migration and proliferation was evaluated by in vitro wound healing assays and sulphorhodamine assays, respectively.
Results
Glioma-derived cells U87-MG displayed lower DKK1 expression compared with bmMSCs. Hypoxia led to an overexpression of DKK1 in bmMSCs and U87-MG when compared to normoxic environment, whereas co-culture of U87-MG with bmMSCs induced the expression of DKK1 in both cell lines. Exogenous recombinant DKK1 inhibited cell migration on all cell lines, but did not have a significant effect on cell proliferation of bmMSCs and glioma cell lines.
Conclusion
In this study, we showed for the first time that the expression of DKK1 was hypoxia dependent in human malignant glioma cell lines. The induction of DKK1 by intracellular crosstalk or hypoxia stimuli sheds light on the intense adaption of glial tumour cells to environmental alterations.









Similar content being viewed by others
References
Akiyama T (2000) Wnt/beta-catenin signaling. Cytokine Growth Factor Rev 11(4):273–282
Bakondi B et al (2009) CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther 17(11):1938–1947
Fedi P et al (1999) Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem 274(27):19465–19472
Forget MA et al (2007) The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer 96(4):646–653
Genetos DC et al (2010) Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem 110(2):457–467
Glinka A et al (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391(6665):357–362
Gregory CA et al (2005) How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci 1049:97–106
Gunn WG et al (2006) A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells 24(4):986–991
Hirata H et al (2011) Wnt antagonist DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation in human renal cell carcinoma. Int J Cancer 128(8):1793–1803
Horwitz EM (2004) Dkk-1-mediated expansion of adult stem cells. Trends Biotechnol 22(8):386–388
Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116(Pt 13):2627–2634
Kazanskaya O, Glinka A, Niehrs C (2000) The role of Xenopus dickkopf1 in prechordal plate specification and neural patterning. Development 127(22):4981–4992
Kimura-Yoshida C et al (2005) Canonical Wnt signaling and its antagonist regulate anterior–posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell 9(5):639–650
Koch S et al (2009) Dkk-1 inhibits intestinal epithelial cell migration by attenuating directional polarization of leading edge cells. Mol Biol Cell 20(22):4816–4825
Kuang HB et al (2009) Dickkopf-1 enhances migration of HEK293 cell by beta-catenin/E-cadherin degradation. Front Biosci 14:2212–2220
Li JJ et al (2009) EphrinA5 acts as a tumor suppressor in glioma by negative regulation of epidermal growth factor receptor. Oncogene 28(15):1759–1768
Ling L, Nurcombe V, Cool SM (2009) Wnt signaling controls the fate of mesenchymal stem cells. Gene 433(1–2):1–7
Mao B et al (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417(6889):664–667
Mikheev AM et al (2004) A functional genomics approach for the identification of putative tumor suppressor genes: Dickkopf-1 as suppressor of HeLa cell transformation. Carcinogenesis 25(1):47–59
Mikheev AM et al (2008) Dickkopf-1 mediated tumor suppression in human breast carcinoma cells. Breast Cancer Res Treat 112(2):263–273
Motaln H et al (2012) Human mesenchymal stem cells exploit the immune response mediating chemokines to impact the phenotype of glioblastoma. Cell Transplant 21(7):1529–1545
Mueller W et al (2005) Mutation analysis of DKK1 and in vivo evidence of predominant p53-independent DKK1 function in gliomas. Acta Neuropathol 109(3):314–320
Niehrs C (2006) Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25(57):7469–7481
Qian J et al (2007) Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood 110(5):1587–1594
Qiao L et al (2008) Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett 269(1):67–77
Qin X et al (2007) Proliferation and migration mediated by Dkk-1/Wnt/beta-catenin cascade in a model of hepatocellular carcinoma cells. Transl Res 150(5):281–294
Schichor C et al (2012) Mesenchymal stem cells and glioma cells form a structural as well as a functional syncytium in vitro. Exp Neurol 234(1):208–219
Shimokawa T et al (2008) Novel human glioma-associated oncogene 1 (GLI1) splice variants reveal distinct mechanisms in the terminal transduction of the hedgehog signal. J Biol Chem 283(21):14345–14354
Shou J et al (2002) Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene 21(6):878–889
Wang J, Shou J, Chen X (2000) Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene 19(14):1843–1848
Zhou XL et al (2010a) Downregulation of Dickkopf-1 is responsible for high proliferation of breast cancer cells via losing control of Wnt/beta-catenin signaling. Acta Pharmacol Sin 31(2):202–210
Zhou Y et al (2010b) Elevated expression of Dickkopf-1 increases the sensitivity of human glioma cell line SHG44 to BCNU. J Exp Clin Cancer Res 29:131
Zhou Y et al (2010c) Analysis of the expression profile of Dickkopf-1 gene in human glioma and the association with tumor malignancy. J Exp Clin Cancer Res 29:138
Acknowledgments
This work was generously supported the SYSTHER/INREMOS projects on Systems Biology Tools development for cell Therapy and Drug Development (Con. No.: 3211-06-000539; 2006-2011), funded by the German and Slovenian Federal Ministries of Education and Research and the SFB 824 (Deutsche Forschungsgemeinschaft). Peng Fu was supported by China Scholarship Council (No. 2009616010).
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work; there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in the manuscript entitled, ‘The expression of Wnt-inhibitor Dickkopf 1 (DKK1) is determined by intercellular crosstalk and hypoxia in human malignant gliomas’.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ke-Tai Guo and Peng Fu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, KT., Fu, P., Juerchott, K. et al. The expression of Wnt-inhibitor DKK1 (Dickkopf 1) is determined by intercellular crosstalk and hypoxia in human malignant gliomas. J Cancer Res Clin Oncol 140, 1261–1270 (2014). https://doi.org/10.1007/s00432-014-1642-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1642-2