Abstract
Children with cerebral palsy (CP) often show executive function (EF) impairments that are key to quality of life. The aim of this study was to assess whether a home-based computerized intervention program improves executive functions (EFs) compared to usual care. Sixty participants (30 females) with CP (8–12 years old) were paired by age, sex, motor ability, and intelligence quotient score and then randomized to intervention and waitlist control groups. The intervention group received a 12-week home-based computerized EF intervention (5 days/week, 30 min/day, total dose 30 h). Core and higher-order EFs were assessed before, immediately after, and 9 months after completing the intervention. The intervention group performed better than the waitlist control group in the three core EFs (immediately and 9 months after the intervention): inhibitory control (F = 7.58, p = 0.13 and F = 7.85, p = 0.12), working memory (F = 8.34, p = 0.14 and F = 7.55, p = 0.13), and cognitive flexibility (F = 4.87, p = 0.09 and F = 4.19, p = 0.08). No differences were found between the groups in higher-order EFs or EF manifestations in daily life.
Conclusions: A home-based computerized EF intervention improved core EFs in children with CP, but further research is needed to identify strategies that allow the transfer of these improvements to everyday life.
Trial registration: NCT04025749 retrospectively registered on 19 July 2019.
What is Known: • One in two children with cerebral palsy has an intellectual impairment. Visual perception and executive functions are the most reported specific cognitive deficits. • The majority of interventions for cerebral palsy focus on motor impairments, but only a few randomized controlled trials have explored the effect of interventions on executive functions. | |
What is New: • A home-based computerized cognitive intervention can improve the core executive functions of children with cerebral palsy. • Short- and long-term effects on core executive functions have been found. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral palsy (CP) is a major cause of physical disability in children, with a median estimated prevalence of 1.6 per 1000 live births [1]. CP is described as a group of permanent disorders that affect the development of movement and posture, causing activity limitations attributed to nonprogressive disturbances that occur in the developing fetal or infant brain and persist throughout life [2]. This motor function impairment is often accompanied by disturbances of sensation, cognition, perception, communication, behavior, and epilepsy [2,3,4].
One in two children with CP has an intellectual impairment [5], which may be more disabling than the motor impairment itself [6, 7]. Visual perception [6] and executive functions (EFs) [8] are the most reported specific cognitive deficits in children with CP.
EFs include a set of complex cognitive skills that work together to direct behavior for decision-making and action planning and play a critical role in behavior and emotional control in daily life [9, 10]. EFs develop throughout childhood and adolescence and are essential for mental and physical health, daily life functioning, and academic achievement [9, 10].
According to Diamond [11], there are three core EFs: inhibitory control, working memory, and cognitive flexibility. Inhibitory control allows individuals to control their attention, behavior, thoughts, and/or emotions to override a strong internal predisposition or external lure. Working memory refers to the ability to hold information in mind and mentally process it. Cognitive flexibility is described as the ability to change perspectives or approaches to a problem and refers to flexibility in adjusting to new demands, rules, or priorities. These three core EFs constitute the fundamental base of higher-order EFs: reasoning, problem solving, and planning [11].
Difficulties in all three core EF domains (inhibitory control, working memory, and flexibility) are found in children with CP [7, 8, 12,13,14,15,16,17,18]. Furthermore, higher-order EF impairments have been reported in individuals with CP [7, 8, 13]. Some of the abovementioned studies used performance-based tests [8, 12,13,14,15,16,17,18,19], and others also used rating scales [7, 8, 15, 19], which are both necessary for the clinical diagnosis of neurological disorders, as they assess different aspects of EFs [9, 20, 21]. Considering that EF deficits have been identified in children with CP and the relationship between these deficits and the quality of life of people with CP [22], there is a clear need for EF interventions targeting people with CP.
The majority of interventions for CP focus on motor impairments [5, 23,24,25], but only a few randomized controlled trials (RCTs) have explored the effect of interventions (multimodal, physical, and cognitive) on EF. Specifically, some studies reported inhibitory control improvements immediately after multimodal [26], physical [19], or cognitive interventions [27]. Only a cognitive intervention showed efficacy in improving working memory immediately postintervention [27]. No previous short-term positive effects after multimodal, physical, or cognitive interventions on the core EF of cognitive flexibility were shown [28]. Only one multimodal study was previously conducted to determine the long-term follow-up effects on core EF outcomes. The results of that study (which did not include a control group) showed improvements in cognitive flexibility 6 months after the intervention [29]. Short-term positive effects of a multimodal intervention have only been found for the higher-order EF of reasoning [30]. Until now, there have been no studies targeting cognitive interventions that attempt to improve all core EF components with the same intervention, testing the effect on higher-order EFs and on daily life performance in children with CP. The present study was aimed at testing whether a home-based computerized cognitive intervention had positive short- and long-term effects on all core and higher-order EFs and manifestations of EF in daily life in children with CP.
Materials and methods
Study design and procedure
A researcher-blinded, matched-pair, randomized waitlist-controlled trial was performed as detailed in the study protocol [31].
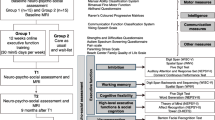
Participants were matched in pairs based on age (8–10.5/ 10.6–12 years), sex, Manual Ability Classification System (MACS) level (I–II/III) [32], and intelligence quotient (IQ) score (< 80/ ≥ 80) [33]. Each of the paired participants was then randomized to the intervention (12-week cognitive intervention) or waitlist control (usual care) group. An EF assessment was carried out at three time points: before (T0, baseline), immediately after (T1, postintervention), and 9 months after (T2, follow-up) completing the intervention, as shown in Fig. 1. After finishing the 9-month follow-up assessment, the intervention was offered to the participants in the waitlist control group.
The current study was retrospectively registered on the 19th of July 2019 at ClinicalTrials.gov (NCT04025749). Ethical approval was obtained from the University of Barcelona’s Institutional Ethics Committee Institutional Review Board (IRB 00003099, assurance number: FWA00004225; http://www.ub.edu/recerca/comissiobioetica.htm) and from Sant Joan de Déu—Barcelona Children’s Hospital Ethics Committee (PIC-45–20). The research was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all parents or legal guardians of the participants, and oral informed consent was obtained from all participants.
Participants
Sixty children diagnosed with CP (30 females; mean age 10.3 years, SD 1.6) were recruited from Sant Joan de Déu-Barcelona Children’s Hospital, Hospital Vall d’Hebron, Fundació ASPACE Catalunya; from a previous study of the research group [22]; and through a webpage created for recruitment purposes. The inclusion criteria were (i) children aged 8–12 years; (ii) children presenting with MACS levels I, II, or III; (iii) children who were able to use an intelligible yes/no response system; (iv) children who were able to understand simple instructions as evaluated by the Screening Test of Spanish Grammar [34]; (v) children who were available to participate in the study for a whole year; and (vi) children who had internet access at home. Children were excluded if they had hearing or visual impairments that precluded neuropsychological assessment and cognitive intervention. A total of 60 participants were determined as the required sample size, as described in the study protocol [31].
Randomization
Participants were randomized using an in-house program written in R by the statistician, which generated the allocation sequence and assigned participants to the intervention or waitlist control group. Once the randomization process was completed, the researcher in charge of the intervention informed the participants’ parents or legal guardians about the group allocation. Participants in the intervention group were informed about the details of the home-based computerized program. The researcher who carried out the assessment remained blinded to the group assignment of each participant throughout the entire study.
Intervention
NeuronUp (www.neuronup.com) is the computerized cognitive intervention that was used in this project, as detailed in the study protocol [31]. Figure 1 shows the intervention structure. The total proposed dose of the direct intervention was 30 h, distributed over 12 weeks, with a total of 120 sessions (15 min every session), namely, 10 sessions per week (2.5 h per week). During the first 6 weeks, the intervention mainly focused on all three core EFs. The higher-order EF intervention, including some social cognition tasks, was introduced after the sixth week. According to the distribution program, the total intervention dose was 20.6 h for the core EF intervention and 9.4 h for the higher-order EFs/social cognition intervention. The EF intervention started at the basic level of difficulty and was gradually adjusted automatically. Manual adjustment of the sessions was necessary in some cases, such as rescheduling sessions missed due to illness, holiday, or homework.
To mainly ensure that the participants received the full dose, the following adherence strategies were used: (1) information strategies: a personalized schedule decorated with pictures related to the children’s interests was delivered, including program instructions, important appointments for neuropsychological assessments, and a special space to record the activities that the children carried out during these 12 weeks. (2) Flexibility: each week, each family would elect 5 days across which to complete ten short sessions; (3) Gaming: the tasks chosen were those with an appearance similar to videogames. (4) Motivational monitoring: during the intervention period, motivational monitoring was carried out by providing personalized immediate messages on platforms such as WhatsApp. Messages were sent weekly to assess motivation and compliance with the previous week’s tasks, which allowed for a minimal standardization of follow-up. After the initial messages, the subsequent messages were always personalized to the children’s preferences and family dynamics. Through these messages, the therapist highlighted positive aspects of the children’s performance to increase motivation and intervention adherence. (5) Expert diploma: all participants were informed that after completing the intervention, they would receive a “NeuronUp’s expert diploma.” The same information strategies, motivational monitoring, and expert diploma were applied to the control group.
Assessment measures
Motor functioning was classified according to the Gross Motor Function Classification System (GMFCS) [35], MACS [32], and Bimanual Fine Motor Function (BFMF) classification [36], and hand function was assessed using the parent-reported Abilhand-Kids scale [37]. Communication skills were classified using the Communication Function Classification System (CFCS) [38] and the Viking Speech Scale (VSS) [39]. Other variables that may have influenced the effect of the intervention were measured by the Bodily Pain and Discomfort Scale of the Child Health Questionnaire (CHQ) [40], Autism Spectrum Screening Questionnaire (ASSQ) [41], Strengths and Difficulties Questionnaire (SDQ) [42], Beach Center Family Quality of Life Scale (fQOL) [43], and Parental Stress Scale (PSS) [44].
Instruments used to measure executive functioning include outcomes of the core EFs (inhibitory control, working memory, and cognitive flexibility) and higher-order EF and their impact on the manifestations of executive functioning in daily life activities. All these instruments were selected considering their reliability and other psychometric properties [31].
Inhibitory control
The Digit Span subtest (WISC-V) was used to assess verbal inhibitory control considering forward, backward, and increasing conditions [45]. The Spatial Span subtest (WNV; Wechsler Nonverbal Scale of Ability) was used to assess visual inhibitory control considering forward and backward conditions [46]. Moreover, the Inhibition index (FDT; Five Digit Test) [47] and the Auditory Attention subtest (NEPSY-II) [48] were used.
Working memory
Verbal working memory was assessed by using the backward conditions of the Digit Span subtests of the WISC-V [45], while the Spatial Span backward condition of the WNV was selected to assess visual working memory [46].
Cognitive flexibility
The Response Set and Word Generation (semantic and initial letter) tests of the NEPSY-II were used to assess cognitive flexibility [48]. Moreover, cognitive flexibility was measured by the Five Digit Test (FDT) [47].
Higher-order EFs
The Tower test from the Delis-Kaplan EF System was employed to measure planning skills [49].
Manifestations of EF in daily life
To assess behavioral manifestations of executive functioning in everyday life, the parent-proxy version of the Behavior Rating Inventory of Executive Function-2 (BRIEF-2) [51] was used. This scale comprises four indices: the Behavioral Regulation index, Emotional Regulation index, Cognitive Regulation index, and Global Index of EF. In this scale, higher T scores indicate poorer performance [51].
Statistical methods
Per-protocol statistical analyses were performed using IBM SPSS v26 (Statistical Package for the Social Sciences, version 26). Graphical representations were performed with R (version 4.1.0; R Core Team, 2021). Neuropsychological raw data were converted to z scores (mean = 0, SD = 1), except for the BRIEF-2 indices, which were converted to T scores (mean = 50, SD = 10), based on normative data corrected by age and sex (Table S1). The Shapiro–Wilk test was used to test each variable’s normality. Summary statistics are reported as the mean (standard deviation), median (minimum–maximum), or frequency (percentage) depending on the measurement scale of the variables analyzed. Several physical (pain), mental (autism symptoms and daily difficulties), and environmental (family quality of life and parental stress) variables were considered as potential covariates [40,41,42,43,44]. Correlations between baseline outcomes and these potential covariates were performed (Pearson’s, Spearman’s, or Kendall’s correlation test depending on the measurement scales), applying Bonferroni’s correction (significance level of p = 0.01). Only potential covariates that were significantly correlated with the baseline outcomes were included as covariates in our models. To test the effectiveness of the intervention, comparisons between the intervention group and waitlist control group postintervention and at the 9-month follow-up were performed by a series of ANCOVAs (analysis of covariance), with baseline assessments used as covariates in all analyses. The statistical assumptions required for the ANCOVAs, such as the normal distribution of the tested variables, were previously checked. Effect size was assessed by means of the partial eta-squared (\({\eta }_{p}^{2}\)) index, considering 0–0.05 as small, 0.06–0.13 as medium, and ≥ 0.14 as large effect sizes [52].
Finally, we performed complementary intention-to-treat (ITT) analysis with R (version 4.1.0; R core Team, 2021), which is available as supplementary material (S4 and S5), to assess the potential bias resulting from the withdrawal of 3 participants. For each given outcome, a longitudinal imputation procedure was applied to the data of individuals who underwent the baseline assessment for that outcome (CopyMean-LOCF procedure; [53, 54]). Then, a series of ANCOVAs including the same covariates as the ANCOVAs applied in the per-protocol analysis were performed for each outcome. The imputation procedure carried out in the present study proved to be optimal when there were monotone missing data (for further details, see 54).
Results
Participants
Enrolment, allocation, and follow-up are reported according to CONSORT guidelines (Fig. S1) [55]. A total of 140 families were informed about the study and screened for inclusion. Of these 140 families, 53 declined the invitation, and 8 were excluded based on the inclusion and exclusion criteria. Subsequently, 79 children eligible for participation were matched. Prior to the beginning of the study, 16 participants were not included (79% retention rate before the intervention). After randomization, the initial sample included 31 participants in the computerized cognitive intervention group and 32 participants in the waitlist control group. One participant in the intervention group dropped out due to family reasons (97% retention rate after the preintervention assessment). Two participants from the waitlist control group declined to participate due to disagreement with the allocation condition (93% retention rate after the preintervention assessment). The total retention rate was 100% (n = 60) during follow-up.
Based on sample size calculation [31] and the withdrawal of 3 participants, the total number of participants that was required to detect changes in the outcome measures was 26 participants in each condition. Thirty participants were included in each group.
The participants’ demographic and clinical characteristics at baseline are presented in Table 1 (mean, standard deviation, interquartile range, number of participants, and percentages). Similarly, the same sample´s descriptive data for potential covariates are presented in Table 2. No significant differences were found between the groups, as shown in Tables 1 and 2. Recruitment took place between November 2017 and December 2020. Postintervention assessments were completed in April 2021, and follow-up assessments were completed in January 2022.
Intervention
From the 30 h (120 sessions) initially planned for the intervention, a mean of 28.35 h (114 sessions) was completed, with a range between a minimum of 26.30 h (106 sessions) and a maximum of 30 h (120 sessions). The lockdown that took place during the first months of the COVID-19 pandemic in Spain affected the families’ organization, preventing them from carrying out the sessions as planned. Specifically, due to this situation, 3 participants in the intervention group had to extend their training period by five additional weeks, achieving, in the end, the same number of sessions and assessments as the rest of the participants. The mean rate of missed sessions was only 5.0% (a minimum rate of 0.0% and a maximum rate of 13%). Missing data in the analyses due to assessment limitations are specified in the supplementary material.
Outcomes
Compared to the waitlist control group, the computerized cognitive intervention group showed higher performance immediately after the intervention and follow-up period in some tasks of the three core EFs outcomes, as reported in Tables S2 and S3. Tables S4 and S5 show the ITT analyses for all outcomes included in the study. All results except those for working memory at the 9-month follow-up were the same for the per-protocol and ITT analyses. The covariates used in each analysis and average scores adjusted for covariates in the model (estimated marginal means) are also indicated.
Inhibitory control
The intervention group performed better in Spatial Span (F = 7.58, p = 0.008, \({\eta }_{p}^{2}\)= 0.13) than the control group postintervention and in Digit Span (WISC-V) (F = 7.85, p = 0.007, \({\eta }_{p}^{2}\)= 0.12) during follow-up assessments, with a medium effect size.
Working memory
The intervention group performed better on the working memory task at postintervention and during follow-up assessments. Specifically, the intervention group performed better on the Spatial Span backward task (WNV; F = 8.34, p = 0.006, \({\eta }_{p}^{2}\) = 0.14; F = 7.55, p = 0.008, \({n}_{p}^{2}\) = 0.13) immediately after the intervention (large effect size; F = 8.34, p = 0.006, \({\eta }_{p}^{2}\) = 0.14) and at the 9-month follow-up (medium effect size; F = 7.55, p = 0.008, \({n}_{p}^{2}\) = 0.13).
Cognitive flexibility
The intervention group performed better on the Response Set task (NEPSY-II) immediately after the intervention (F = 4.87, p = 0.032, \({\eta }_{p}^{2}\) = 0.09) and at the 9-month follow-up (F = 4.19, p = 0.046, \({\eta }_{p}^{2}\) = 0.08) assessments, with medium effect sizes.
Higher-order EFs
There were no differences between the intervention and waitlist control groups in higher-order EFs (Tower, D-KEFS) and behavioral manifestations of executive functioning in everyday life (BRIEF-2) outcomes immediately and 9 months after the intervention.
Graphical representations
The graphical representations of the results are presented in Figs. 2, 3, and 4 and Figs. S2 and S3. Boxes represent the estimated marginal differences (differences between the groups’ estimated marginal means in Tables S2 and S3) between the intervention and waitlist control groups immediately after the intervention (T1) and at the 9-month follow-up (T2). The intervention group showed better performance on the majority of the cognitive domains than the waitlist control group (positive differences shown in Figs. 2, 3, and 4 and Fig. S2; negative differences shown in Fig. S3), although not all reached significance (dark gray boxes). The graphical representation also shows two different patterns in some measures. First, there was an ascending pattern in which changes were higher at the 9-month follow-up than immediately postintervention, as seen, for example, in the Digit Span (WISC-V) plot (Fig. 2). Second, there was a descending pattern in which the highest differences were in the postintervention assessment compared to the 9-month follow-up assessment, as seen, for example, in the Spatial Span (WNV) plot (Fig. 2).
Graphical representation of differences between intervention and waitlist groups in inhibitory control. Notes: dark gray box (significant differences between the intervention and waitlist group); light gray (no significant differences). Estimated marginal differences (estimated marginal mean of the intervention group − estimated marginal mean of the waitlist control group) above zero indicate that the intervention group has better performance than the waitlist group. Whiskers correspond to the 95% CIs for the marginal differences. Abbreviations: T1, postintervention; T2, 9-month follow-up after the intervention; FDT, Five Digit Test; NEPSY-II, a Developmental Neuropsychological Assessment, Second Edition; WISC-V, Wechsler Intelligence Scale for Children, Fifth Edition; WNV, Wechsler Nonverbal Scale of Ability
Graphical representation of differences between intervention and waitlist groups in working memory. Notes: dark gray box (significant differences between the intervention and waitlist group); light gray (no significant differences). Estimated marginal differences (estimated marginal mean of the intervention group − estimated marginal mean of the waitlist control group) above zero indicate that the intervention group has better performance than the waitlist group. Whiskers correspond to the 95% CIs for the marginal differences. Abbreviations: T1, postintervention; T2, 9-month follow-up after intervention; WISC-V, Wechsler Intelligence Scale for Children, Fifth Edition; WNV, Wechsler Nonverbal Scale of Ability
Graphical representation of differences between intervention and waitlist groups in cognitive flexibility graphical representation. Notes: dark gray box (significant differences between the intervention and waitlist group); light gray (no significant differences). Estimated marginal differences (estimated marginal mean of the intervention group − estimated marginal mean of the waitlist control group) above zero indicate that the intervention group has better performance than the waitlist group. Box sizes represent the magnitude of the estimated differences (i.e., areas are proportional to the corresponding estimated effect), whereas wWhiskers correspond to the 95% CIs for the marginal differences. Abbreviations: T1, postintervention; T2, 9-month follow-up after intervention; FDT, Five Digit Test; NEPSY-II, a Developmental Neuropsychological Assessment, Second Edition
Discussion
The main findings of this study suggest that a computerized intensive and progressively challenging EF home intervention improved core EFs performance (inhibitory control, working memory, and cognitive flexibility) in children with CP. Core EF differences between the groups in some tasks were also demonstrated at the 9-month follow-up after completing the intervention. To our knowledge, this is the first study to demonstrate that a 30-h cognitive intervention improved performance in some tasks covering all core EFs.
This positive effect in the core EFs may translate to large and significant improvements in behavior, attention, thoughts, and/or emotional control [56]. Specifically, improvements in inhibitory control meant that children’s ability to focus on what they chose increased, instead of focusing on making better decisions about what was more appropriate or needed, by suppressing their attention to other stimuli [11]. These improvements align with previous RCTs with a low risk of bias that showed significant improvements in inhibitory control with multimodal and cognitive interventions immediately after the interventions [28]. Long-term effects, however, were not explored in these studies. Working memory changes, reported in the present study, implied that children’s capacity to hold information in mind for a short time and mentally process it increased [11]. Consistent with our results, Di Lieto et al. [27] found that children with CP presented significant improvements in working memory immediately after receiving a 5-week computerized cognitive intervention but not at follow-up [21]. Finally, cognitive flexibility improvements suggested that the intervention increased the children’s ability to be more flexible between different tasks. This flexibility may allow children to adjust to changing demands or priorities, to admit what is wrong, and to take advantage of sudden unexpected opportunities [11]. Previous studies have not shown beneficial effects on cognitive flexibility after multimodal or cognitive interventions [26, 27, 56]. Although Mak et al. [26] in 2018 did not find improvements in cognitive flexibility immediately after the intervention, delayed effects were found 6 months later [29].
Improvements in higher-order EFs have only been found in reasoning using a reality-based rehabilitation program for children with CP [30]. In the present study, we did not find significant differences in higher-order EFs related to planning. Previous RCTs with a low risk of bias also showed no improvements using the same planning measure (Tower test) as that used in the present study [56]. Piovesana et al. [56] discussed that their negative results might be because the cognitive challenges of their multimodal intervention were not focused on EFs. In the present study, the intervention specifically targeted EFs. We therefore concluded that the lack of beneficial effects on higher-order EF outcomes could be due to insufficient training time dedicated to higher-order EF tasks. Specifically, from the 30-h total dose in the present study, only 33% of the dose corresponded to tasks targeting higher-order EFs. Future studies should explore whether increasing the dose of higher-order EF training results in significant improvements in this domain.
Identifying the optimal dose is a key factor to guarantee maximum adherence to treatment. Such an optimal dose allowing positive results in core EFs is highly variable in previous studies. For example, Ahn et al. [19] found that the optimal intervention dose was 21.3 h, reported as a mean. In the study by Di Lieto et al. [27], the intervention dose ranged from 8 to 18 h. Finally, Mak et al. [26] reported 12.5 h as the mean intervention dose. In the present study, we allowed flexibility for reaching the total dose (each week, the participants could elect the 5 days across which to complete the ten sessions, and several motivational strategies were used to improve participant and family engagement). These aspects allowed a small variability in the total dose achieved (26 to 30 h), which represents an attrition rate of only 5%.
Once the optimal dose has been identified, computerized interventions such as the one in this study may also be key to achieving it. Computerized cognitive interventions can be beneficial for children with various conditions, including CP, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and learning difficulties [57, 58]. These interventions improved cognitive skills, such as working memory and flexibility skills, in children with ADHD or ASD [59, 60]. A systematic review of children with and without neuropsychological disorders also stated that this type of intervention is effective in improving cognitive skills [57]. Additionally, creating EF intervention opportunities outside of the rehabilitation center setting could provide more parental agency in the rehabilitation process and a more collaborative and supportive approach to an intervention in the natural environment, leading to greater compliance with programs, reducing stress levels, and improving outcomes.
Our results did not prove positive effects on manifestations of executive functioning in daily life, assessed with a rating scale that measures core and higher-order EFs. The results of this study reinforce the idea that performance-based tests and rating scales assess different EF aspects [9, 20]. Both instruments are complementary and should be used for assessing EFs. In this way, this could help to characterize the impairment and consequently prove the right support for patients and caregivers. Overall, our results are consistent with previous studies in other child populations related to EFs, such as populations with ASD, ADHD, or learning difficulties, in which transfer effects on manifestations of EF in daily life have not been reported [9, 20, 21]. Thus, further research is needed to clarify intervention characteristics and the assessment tools used to check intervention effects. These studies may also propose interventions that include face-to-face interventions and specific professional advice that allow the transfer of changes to manifestations of EF in daily life.
A limitation of the present study is that it did not include children across all MACS levels. Only participants at levels I–III were included to homogenize the characteristics of the sample and the effective time of the cognitive intervention among participants. Moreover, adaptations in the cognitive assessment were applied for one participant with vision impairment. In this participant, a computerized, instead of paper, version of the FDT was used. Additionally, we did not include an active control group because almost all cognitive tasks imply some level of EF. Other factors might be of interest to consider in cognitive intervention effectiveness, such as nutritional status [61, 62] or sleep disorders [63]. In addition, some families needed three more weeks (15 weeks instead of 12) to reach the total dose due to the COVID-19 pandemic. Finally, the pandemic might also have influenced, to some degree, children’s responses to treatment due to the potential decrease in their general health as a result of the disruption in health and rehabilitation services [64].
Conclusions
Our results indicate that a home-based computerized EF intervention, together with motivational monitoring strategies that enhance adherence, can improve the core EFs of children with CP for at least 9 months postintervention. This intervention could be complementary to conventional face-to-face therapies to intensively stimulate cognitive functioning in children with CP. Further research is needed to identify strategies that allow the improvements to be transferred to everyday life and to test this intervention across the full spectrum of severity that people with CP can present.
Data availability
Online resources are available. All data relevant to the study are included in the article or uploaded as supplementary information. Original data are available from the corresponding author upon request.
References
McIntyre S, Goldsmith S, Webb A, Ehlinger V, Hollung SJ, McConnell K et al (2022) Global prevalence of cerebral palsy: a systematic analysis. Dev Med Child Neurol 64(1494–506). https://doi.org/10.1111/dmcn.15346
Rosenbaum P, Paneth N, Levinton A, Goldstein M, Bax M, Damiano D et al (2007) A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol 49(8–14)
Graham HK, Rosenbaum P, Paneth N, Dan B, Lin J-P, Damiano DL et al (2016) Cerebral palsy. Nat Rev Dis Primers 2(1). https://doi.org/10.1038/nrdp.2015.82
Straub K, Obrzut JE (2009) Effects of cerebral palsy on neuropsychological function. J Dev Phys Disabil 21(2):153–167. https://doi.org/10.1007/S10882-009-9130-3
Novak I, Mcintyre S, Morgan C, Campbell L, Dark L, Morton N et al (2013) A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol 55(10):885–910. https://doi.org/10.1111/dmcn.12246
Ego A, Lidzba K, Brovedani P, Belmonti V, Gonzalez-Monge S, Boudia B et al (2015) Visual-perceptual impairment in children with cerebral palsy: a systematic review. Dev Med Child Neurol 57(57):46–51. https://doi.org/10.1111/dmcn.12687
Påhlman M, Gillberg C, Himmelmann K (2021) Autism and attention-deficit/hyperactivity disorder in children with cerebral palsy: high prevalence rates in a population-based study. Dev Med Child Neurol 63(3). https://doi.org/10.1111/dmcn.14736
Stadskleiv K, Jahnsen R, Andersen GL, von Tetzchner S (2017) Neuropsychological profiles of children with cerebral palsy. Dev Neurorehabil 21(2):108–120. https://doi.org/10.1080/17518423.2017.1282054
Anderson PJ, Reidy N (2012) Assessing executive function in preschoolers. Neuropsychol Rev 22(4):345–360. https://doi.org/10.1007/s11065-012-9220-3
Diamond A (2016) Why improving and assessing executive functions early in life is critical. Executive function in preschool-age children: integrating measurement, neurodevelopment, and translational research. (11–3):11–43. https://doi.org/10.1037/14797-002
Diamond A (2013) Executive functions. Annu Rev Psychol 64(1):135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Bartonek Å, Piccardi L, Guariglia C (2020) Topographical working memory in children with cerebral palsy. J Mot Behav 53(22–208):1–9. https://doi.org/10.1080/00222895.2020.1748861
Bodimeade HL, Whittingham K, Lloyd O, Boyd RN (2013) Executive function in children and adolescents with unilateral cerebral palsy. Dev Med Child Neurol 55(10):926–933. https://doi.org/10.1111/dmcn.12195
Bottcher L (2010) Children with spastic cerebral palsy, their cognitive functioning, and social participation: a review. Child Neuropsychol 16(3):209–228. https://doi.org/10.1080/09297040903559630
Di Lieto MC, Brovedani P, Pecini C, Chilosi AM, Belmonti V, Fabbro F et al (2017) Spastic diplegia in preterm-born children: executive function impairment and neuroanatomical correlates. Res Dev Disabil 61(61):116–126. https://doi.org/10.1016/j.ridd.2016.12.006
Freire TC, Osório AAC (2019) Executive functions and drawing in young children with cerebral palsy: comparisons with typical development. Child Neuropsychol 26(5):635–648. https://doi.org/10.1080/09297049.2019.1694648
Gagliardi C, Tavano A, Turconi AC, Pozzoli U, Borgatti R (2011) Sequence learning in cerebral palsy. Pediatr Neurol 44(3):207–213. https://doi.org/10.1016/j.pediatrneurol.2010.10.004
Pirila S, van der Meere JJ, Rantanen K, Jokiluoma M, Eriksson K (2011) Executive functions in youth with spastic cerebral palsy. J Child Neurol 26(7):817–821. https://doi.org/10.1177/0883073810392584
Ahn B, Joung Y-S, Kwon J-Y, Lee DI, Oh S, Kim B-U et al (2021) Effects of equine-assisted activities on attention and quality of life in children with cerebral palsy in a randomized trial: examining the comorbidity with attention-deficit/hyperactivity disorder. BMC Pediatrics 21(1). https://doi.org/10.1186/s12887-021-02597-0
MCauley T, Chen S, Goos L, Schachar R, Crosbie J (2010) Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function?. J Int Neuropsychol Soc 16(3):495–505. https://doi.org/10.1017/S1355617710000093
Miranda A, Berenguer C, Roselló B, Baixauli I, Colomer C (2017) Social cognition in children with high-functioning autism spectrum disorder and attention-deficit/hyperactivity disorder. Associations with Executive Functions. Front Psychol 8(1035). https://doi.org/10.3389/fpsyg.2017.01035
Blasco M, García-Galant M, Laporta-Hoyos O, Ballester-Plané J, Jorba-Bertran A, Caldú X, Miralbell J, Alonso X, Meléndez-Plumed M, Toro-Tamargo E, Gimeno F, Pueyo R (2023) Factors related to quality of life in children with cerebral palsy. Pediatr Neurol 141:101–108. https://doi.org/10.1016/j.pediatrneurol.2023.01.005
Fandim JV, Saragiotto BT, Porfírio GJM, Santana RF (2020) Effectiveness of virtual reality in children and young adults with cerebral palsy: a systematic review of randomized controlled trial. Braz J Phys Ther 25(2). https://doi.org/10.1016/j.bjpt.2020.11.003
Jackman M, Sakzewski L, Morgan C, Boyd RN, Brennan SE, Langdon K et al (2021) Interventions to improve physical function for children and young people with cerebral palsy: international clinical practice guideline. Dev Med Child Neurol 64(5). https://doi.org/10.1111/dmcn.15055
Novak I, Morgan C, Fahey M, Finch-Edmondson M, Galea C, Hines A et al (2020) State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol NeurosciRep 20(2). https://doi.org/10.1007/s11910-020-1022-z
Mak C, Whittingham K, Cunnington R, Boyd RN (2018) Effect of mindfulness yoga programme MiYoga on attention, behavior, and physical outcomes in cerebral palsy: a randomized controlled trial. Dev Med Child Neurol 60(9):922–932. https://doi.org/10.1111/dmcn.13923
Di Lieto MC, Pecini C, Brovedani P, Sgandurra G, Dell’Omo M, Chilosi AM et al (2021) Adaptive working memory training can improve executive functioning and visuo-spatial skills in children with pre-term spastic diplegia. Front Neurol 11(601148). https://doi.org/10.3389/fneur.2020.601148
Blasco M, García-Galant M, Berenguer-González A, Caldú X, Arqué M, Laporta-Hoyos O et al (2022) Interventions with an impact on cognitive functions in cerebral palsy: a systematic review. Neuropsychol Rev (1–27). https://doi.org/10.1007/s11065-022-09550-7
Mak C, Whittingham K, Cunnington R, Chatfield M, Boyd RN (2020) Six-month follow-up of a mindfulness yoga program, MiYoga, on attention, executive function, behavior and physical outcomes in cerebral palsy. Disabil Rehabil 44(6):1–7. https://doi.org/10.1080/09638288.2020.1783582
Aran OT, Şahin S, Köse B, Ağce ZB, Kayihan H (2019) Effectiveness of the virtual reality on cognitive function of children with hemiplegic cerebral palsy. Int J Rehabil Res 43(1):1. https://doi.org/10.1097/MRR.0000000000000378
García-Galant M, Blasco M, Reid L, Pannek K, Leiva D, Laporta-Hoyos O et al (2020) Study protocol of a randomized controlled trial of home-based computerized executive function training for children with cerebral palsy. BMC Pediatrics 20(1). https://doi.org/10.1186/s12887-019-1904-x
Eliasson A, Krumlinde-Sundholm L, Rösblad B, Beckung E, Arner M, Öhrvall A et al (2006) The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol 48(7). https://doi.org/10.1017/S0012162206001162
Raven JC, Court JJ, Raven J (2011) Raven matrices progresivas: CPM color, SPM general. TEA ediciones
Toronto AS (1973) Screening test of Spanish grammar. Northwestern University Press, Evenston, IL
Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH (2008) Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol 50(10):744–750. https://doi.org/10.1111/j.1469-8749.2008.03089.x
Elvrum A-KG, Beckung E, Sæther R, Lydersen S, Vik T, Himmelmann K (2016) Bimanual capacity of children with cerebral palsy: intra- and interrater reliability of a revised edition of the bimanual fine motor function classification. Phys Occup Ther Pediatr 37(3):239–51. https://doi.org/10.1080/01942638.2016.1185507
Arnould C, Penta M, Renders A, Thonnard J-L (2004) ABILHAND-Kids Neurology 63(6):1045–1052. https://doi.org/10.1212/01.wnl.0000138423.77640.37
Hidecker MJC, Paneth N, Rosenbaum PL, Kent RD, Lillie J, Eulenberg JB et al (2011) Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev Med Child Neurol 53(8):704–710. https://doi.org/10.1111/j.1469-8749.2011.03996.x
Pennington L, Virella D, Mjøen T, da Graça AM, Murray J, Colver A et al (2013) Development of the Viking Speech Scale to classify the speech of children with cerebral palsy. Research in Developmental Disabilities [Internet] 34(10):3202–3210. https://doi.org/10.1016/j.ridd.2013.06.035
Landgraf JM, Abetz L, Ware JE (1999) Child Health Questionnaire (CHQ): a user’s manual. Landgraf & Ware
Ehlers S, Gillberg C, Wing L (1999) A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord 29(2):129–141. https://doi.org/10.1023/a:1023040610384
Goodman R (1997) The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 38(5):581–586. https://doi.org/10.1111/j.1469-7610.1997.tb01545.x
Hoffman L, Marquis J, Poston D, Summers JA, Turnbull A (2006) Assessing family outcomes: psychometric evaluation of the Beach Center Family Quality of Life Scale. J Marriage Fam 68(4):1069–1083
Berry JO, Jones WH (1995) The Parental Stress Scale: initial psychometric evidence. J Soc Pers Relat 12(3):463–472. https://doi.org/10.1177/0265407595123009
Wehsler D, Raiford SE, Holdnack JA (2015) WISC-V: Escala de inteligencia de Wechsler para niños-V. Pearson. Pearson
Wechsler D, Naglieri JA (2006) WNV: Wechsler Nonverbal Scale of Ability. Psych Corporation, Psych Corporation
Sedó MA (2007) Five Digits Test. TEA ediciones
Korkman M (2014) Batería Neuropsicológica, NEPSY-II. Pearson
Delis DC, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive Function System. PsycTESTS Dataset. Psychological Corp.
Hendrickson NK, McCrimmon AW (2018) Test review: Behavior Rating Inventory of Executive Function®, Second Edition (BRIEF®2) by Gioia, G. A., Isquith, P. K., Guy, S. C., & Kenworthy, L. Can J Sch Psychol 34(1):73–8
Gioia GA, Isquith PK, Guy SC, Marano A, Al E (2016) BRIEF 2 Behavior Rating Inventory of Executive Function - Second Edition - Manuale. Firenze Hogrefe
Cohen J (1992) A power primer. Psychol Bull 112(1). https://doi.org/10.1037//0033-2909.112.1.155
Genolini C, Écochard R, Jacqmin-Gadda H (2013) Copy Mean: a new method to impute intermittent missing values in longitudinal studies. Open J Stat 03(04):26–40. https://doi.org/10.4236/ojs.2013.34A004
Genolini C, Lacombe A, Écochard R, Subtil F (2016) CopyMean: a new method to predict monotone missing values in longitudinal studies. Comput Methods Programs Biomed 132(1):29–44. https://doi.org/10.1016/j.cmpb.2016.04.010
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 1(2):c332–c342. https://doi.org/10.1136/bmj.c332
Piovesana AM, Ross S, Lloyd O, Whittingham K, Ziviani J, Ware RS et al (2017) Randomized controlled trial of a web-based multi-modal therapy program for executive functioning in children and adolescents with unilateral cerebral palsy. Disabil Rehabil 39(20):2021–2028. https://doi.org/10.1080/09638288.2016.1213899
Oldrati V, Corti C, Poggi G, Borgatti R, Urgesi C, Bardoni A (2020) Effectiveness of computerized cognitive training programs (CCTP) with game-like features in children with or without neuropsychological disorders: a meta-analytic investigation. J Autism Dev Disord 50(8):2921–2935. https://doi.org/10.1007/s11065-020-09429-5
Khan K, Hall CL, Davies EB, Hollis C, Glazebrook C (2019) The effectiveness of web-based interventions delivered to children and young people with neurodevelopmental disorders: systematic review and meta-analysis. J Med Internet Res 21(11):e13478. https://doi.org/10.2196/13478
Westwood SJ, Parlatini V, Rubia K et al (2023) Computerized cognitive training in attention-deficit/hyperactivity disorder (ADHD): a meta-analysis of randomized controlled trials with blinded and objective outcomes. Mol Psychiatry. https://doi.org/10.1038/s41380-023-02000-7
Cavalli G, Galeoto G, Sogos C, Berardi A, Tofani M (2022) The efficacy of executive function interventions in children with autism spectrum disorder: a systematic review and meta-analysis. Expert Rev Neurother 22(1):77–84. https://doi.org/10.1080/14737175.2022.2011215
Sharawat IK, Ramachandran A, Panda PK, Kumar V, Bhat NK (2023) Prevalence, severity, and predictors of malnutrition in Indian children with cerebral palsy and their impact on health-related quality of life. Eur J Pediatr. https://doi.org/10.1007/s00431-023-04930-4
Moss BG, Yeaton WH (2021) Young children’s weight trajectories and associated risk of poor neuropsychological outcomes: results from the contemporary reading skills study. J Pediatr Psychol 46(2):171–182. https://doi.org/10.1093/jpepsy/jsaa099
Hulst RY, Gorter JW, Voorman JM, Kolk E, Van Der Vossen S, Visser-Meily JM, ... Verschuren O (2021) Sleep problems in children with cerebral palsy and their parents. Dev Med Child Neurol 63(11):1344–1350. https://doi.org/10.1111/dmcn.14920
Cankurtaran D, Tezel N, Yildiz SY, Celik G, Unlu AE (2021) Evaluation of the effects of the COVID-19 pandemic on children with cerebral palsy, caregivers’ quality of life, and caregivers’ fear of COVID-19 with telemedicine. Ir J Med Sci 190(4):1473–1480. https://doi.org/10.1007/s11845-021-02622-2
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross HE, Elger CE et al (2014) A practical clinical definition of epilepsy. Epilepsia 55(4):475–482. https://doi.org/10.1111/epi.12550
Acknowledgements
We would like to thank all the children and parents who participated in this study.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This project was supported by the Ministerio de Economía y Competitividad (PSI2016–75979-R AEI/FEDER, UE). This project was also supported by the Agència de Gestió d’Ajuts Universitaris i de Recerca from Generalitat de Catalunya (2017SGR0748, 2021SGR00801) and related to “Evidence-based consensus guidelines for neuropsychological assessment of people with severe and dyskinetic cerebral palsy” supported by the Agencia Estatal de Investigación (PID2020-117163RB-I00/AEI/10.13039/ 501100011033). María García-Galant received a research grant from Generalitat de Catalunya (grant code FI-SDUR2020). Montse Blasco received a research grant from Universitat de Barcelona (grant code APIF_2018–2019). Roslyn Boyd is supported by National Health and Medical Research Council (MHMRC) of Australia Research Fellowship (RB 1037220).
Author information
Authors and Affiliations
Contributions
RP provided supervision throughout the study as lead investigator. MG-G and MB are responsible for the ethics application and reporting. EP-B, JC-M, and XA were responsible for patient selection. MG-G, MB, and AB-G were responsible for recruiting and data collection. DL takes on a lead role in the data and statistical analysis management, with the support of MG-G, MB, and PM-S. MG-G takes a lead role in preparing the publication. RP, MB, OL-H, and JB-P contributed to the preparation of the publication. XC, JM, and RNB contributed advice during the study in their specific fields. MG-G drafted the final version of this manuscript, while all authors critically reviewed and approved the final version.
Corresponding author
Ethics declarations
Ethical approval
The current study was retrospectively registered the 19th of July 2019 in ClinicalTrials.gov (NCT04025749). Ethical approval was obtained from the University of Barcelona’s Institutional Ethics Committee, Institutional Review Board (IRB 00003099, assurance number: FWA00004225; http://www.ub.edu/recerca/comissiobioetica.htm), and from Sant Joan de Déu-Barcelona Children’s Hospital Ethics Committee (PIC-45–20). The research was conducted in accordance with the Helsinki Declaration.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Galant, M., Blasco, M., Laporta-Hoyos, O. et al. A randomized controlled trial of a home-based computerized executive function intervention for children with cerebral palsy. Eur J Pediatr 182, 4351–4363 (2023). https://doi.org/10.1007/s00431-023-05072-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05072-3