Abstract
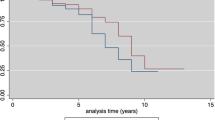
Vaccination should be timed to take into account the potential interference of maternal antibodies. The purpose of this study was to determine the persistence of maternally acquired antibodies to hepatitis A and varicella zoster in a group of healthy infants between 6 and 24 months of age. These infants were divided into four groups according to the age at the time of follow-up visits. The study group consisted of infants who were brought to the 6-month follow-up visit (group 1, n = 100), 12-month follow-up visit (group 2, n = 99), 18-month follow-up visit (group 3, n = 59), and 24-month follow-up visit (group 4, n = 59). Hepatitis A, varicella IgG, and IgM antibodies were analyzed qualitatively. Hepatitis A IgG seropositivity was determined as 71 % in group 1, 41.4 % in group 2, 0 % in group 3, and 8.5 % in group 4 (p < 0.001). Varicella IgG seropositivity was found to be 5 % in group 1, 4 % in group 2, 4 % in group 3, and 1 % in group 4 (p > 0.05).
Conclusion: We found that maternal hepatitis A antibodies in children disappear between 12 and 18 months, whereas maternal varicella antibodies substantially diminish following the sixth month. Therefore, the vaccination timing should be based on factors such as the interference of maternal antibodies, disease susceptibility period, and immune maturation.


Similar content being viewed by others
Abbreviations
- CMIA:
-
Chemiluminescence microparticle immune assay
- DNA:
-
Deoxyribonucleic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- FDA:
-
Food and drug administration
- HAV:
-
Hepatitis virus type A
- Ig:
-
Immunoglobulin
- IgM:
-
Immunoglobulin M
- IgG:
-
Immunoglobulin G
- VZV:
-
Varicella-zoster virus
References
Alabaz D, Aksaray N, Alhan E, Yaman A (2005) Decline of maternal hepatitis A antibodies during the first 2 years of life infants born in Turkey. Am J Trop Med Hyg 73:457–459
Arvin AM (1996) Varicella-zoster virus. Clin Microbiol Rev 9:361–381
Brinkhof MWG, Mayorga O, Bock J, Heininger U, Herzog C (2013) Kinetics of maternally acquired anti-hepatitis A antibodies: prediction of waning based on maternal or cord blood antibody levels. Vaccine 31:1490–1495
Ceyhan M, Yıldırım I, Kurt N et al (2008) Differences in hepatitis A seroprevalence among geographical regions in Turkey: a need for regional vaccination recommendations. J Viral Hepat 15:S69–S72
De Silvestri A, Avanzini MA, Terulla V et al (2002) Decline of maternal hepatitis A virus antibody levels in infants. Acta Paediatr 91:882–884
Dhillon S, Curran MP (2008) Live attenuated measles, mumps, rubella, and varicella zoster virus vaccine (Priorix-Tetra). Paediatr Drugs 10:337–347
FitzSimons D, Hendrickx G, Vorsters A, Van Damme P (2010) Hepatitis A and E: update on prevention and epidemiology. Vaccine 28:583–588
Gans HA, Maltonado YA (2013) Loss of passively acquired maternal antibodies in highly vaccinated populations: an emerging need to define the ontogeny of infant immune responses. J Infect Dis 208:1–3
Glezen WP (2003) Effect of maternal antibodies on the infant immune response. Vaccine 21:3389–3392
Hadinegoro SR, Hindra IS, Han HH, Gatchalian S, Bock HL (2009) Reactogenicity and immunogenicity of a live-attenuated refrigerator-stable varicella vaccine (OKA strain) in healthy seronegative subjects age 10 months to 12 years. Southeast Asian J Trop Med Public Health 40:991–999
Heininger U, Seward JF (2006) Varicella. Lancet 368:1365–1376
Heininger U, Desgrandchamps D, Schaad UB (2006) Seroprevalence of varicella-zoster virus IgG antibodies in Swiss children during the first 16 months of age. Vaccine 24:3258–3260
Jacobsen KH, Koopman JS (2004) Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect 132:1005–1022
Kovarik J, Siegrist CA (1998) Optimization of vaccine responses in early life: the role of delivery systems and immunomodulators. Immunol Cell Biol 76:222–236
Letson GW, Shapiro CN, Kuehn D et al (2004) Effect of maternal antibody on immunogenicity of hepatitis A vaccine in infants. J Pediatr 144:327–332
Leuridan E, Hens N, Hutse V, Aerts M, Damme PV (2011) Kinetics of maternal antibodies against rubella and varicella in infants. Vaccine 29:2222–2226
Lieberman JM, Chang S-J, Partridge S et al (2002) Kinetics of maternal hepatitis A antibody decay in infants: implications for vaccine use. Pediatr Infect Dis J 21:347–348
Marin M, Watson TL, Chaves SS et al (2008) Varicella among adults: data from an active surveillance project, 1995—2005. J Infect Dis 197:S94
Palfi M, Selbing A (1998) Placental transport of maternal immunoglobulin G. Am J Reprod Immunol 39:24–26
Pinquier D, Gagneur A, Balu L, Brissaud O (2009) Prevalence of anti-varicella-zoster virus antibodies in French infants under 15 months of age. Clin Vaccine Immunol 16:484–487
Saji F, SamejimaY KS, Koyama M (1999) Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod 4:81–89
Seward JF (2001) Update on varicella. Pediatr Infect Dis J 20:619–621
Sharapov M, Bulkow LR, Negus SE, Philip R (2012) Persistence of hepatitis A vaccine induce seropositivity in infants and young children by maternal antibody status: 10-year follow up. Hepatology 56:516–522
Siegrist CA (2003) Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21:3406–3412
Siegrist CA (2007) The challenges of vaccine responses in early life: selected examples. J Comp Pathol 137:S4–S9
Van der Zwet WC, Vandenbroucke-Grauls CMJE, van Elburg RM, Cranendonk A, Zaaijer HL (2002) Neonatal antibody titers against varicella-zoster virus in relation to gestational age, birth weight, and maternal titer. Pediatrics 109:79–85
Vidor E (2007) Vaccination of newborns against hepatitis A in the presence of maternally derived antibodies. J Comp Pathol 137:S42–S45
Waaijenborg S, Hahne SJM, Mollema L et al (2013) Waning of maternal antibodies against measles, mumps, rubella, and varicella in communication with contrasting vaccination coverage. J Infect Dis 208:10–16
Wasley A, Fiore A, Bell BP (2006) Hepatitis A in the era of vaccination. Epidemiol Rev 28:101–111
Wasley A, Feinstone SM, Bell BP (2010) Hepatitis A virus. In: Mandell GL, Bennett JE, Dolin R (eds) Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 7th edn. Churchill Livingstone Elsevier, Philadelphia, pp 2367–2387
Whitley RJ (2010) Varicella-zoster virus. In: Mandell GL, Bennett JE, Dolin R (eds) Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 7th edn. Churchill Livingstone Elsevier, Philadelphia, pp 1963–1969
World Health Organization (2000) WHO position paper on hepatitis A vaccines. Wkly Epidemiol Rec 75:38–44
Wutzler P, Färber I, Wagenpfeil S, Bisanz H, Tischer A (2001) Seroprevalence of varicella-zoster virus in the German population. Vaccine 20:121–124
Acknowledgments
This study is supported by Ankara University Research Fund (Project code: 12B3330014).
Conflicts of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Nadal
Revisions received: 01 August 2014 / 11 October 2014 / 16 December 2014
Rights and permissions
About this article
Cite this article
Begde, F., Orhon, F.S., Gerceker, D. et al. Determining the persistence of maternally acquired antibodies to hepatitis A and varicella zoster during the first 2 years of life in Turkey. Eur J Pediatr 174, 883–890 (2015). https://doi.org/10.1007/s00431-014-2484-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-014-2484-2