Abstract
Background
The severity of childhood gastroenteritis is generally believed to be age-related rather than aetiology-related. Rotavirus-induced gastroenteritis is more severe than gastroenteritis caused by other enteric pathogens and is also age-related. We thus addressed the question of whether the increased severity of rotavirus-induced gastroenteritis is related to age or to features intrinsic to the agent.
Study design
In this multicentre, hospital-based, prospective survey, we evaluated the severity of diarrhoea in rotavirus-positive and rotavirus-negative children up to 4 years of age. Severity was assessed with a score in four groups of age-matched children.
Results
Rotavirus was detected in 381 of 911 children. Disease severity was evaluated in 589 cases for which clinical data were complete. The rotavirus-positive and rotavirus-negative groups differed with regards to diarrhoea duration, hospital stay, degree of dehydration and the number of episodes of vomiting. Gastroenteritis was more severe in rotavirus-positive than in rotavirus-negative children. In contrast, none of the main severity parameters differed in the four age groups, irrespective of the presence of rotavirus.
Conclusions
These data provide the evidence that aetiology and not age determines diarrhoeal severity. The demonstration that diarrhoea was more severe in rotavirus-positive children supports the need for a rotavirus vaccine and for studies that address the duration of vaccine protection.
Similar content being viewed by others
Introduction
It is generally believed that, irrespective of aetiology, infants and younger children are at greater risk of severe gastroenteritis than older children because of their immature homeostatic fluid mechanisms and immune response [8, 13]. Rotavirus is the most frequent agent cause of childhood diarrhoea worldwide and rotavirus-induced gastroenteritis is more severe than that caused by other enteric pathogens. Rotavirus is, therefore, responsible for a substantial number of hospitalisations [10, 12, 23, 27]. Since rotavirus has a striking age-related distribution, peaking in infants from 6 to 24 months old [19, 26], it is not clear whether its increased severity is related to age or to features intrinsic to the agent.
It has been estimated that a vaccine would prevent as many as 3.5 million cases annually among children below 5 years of age and 50,000 hospitalisations each year in the USA [32]. In 1998, an anti-rotavirus vaccine became commercially available and the Advisory Committee on Immunization Practices (ACIP) recommended its use [4]. However, it was soon withdrawn because it was implicated in intestinal intussusception [5, 6, 22]. New vaccines are under evaluation [25, 31]. However, before implementing a routine immunisation programme against rotavirus infection, the magnitude of the problem and the expected benefits of a vaccine must be assessed.
The aim of this multicentre, hospital-based, prospective survey was to test the hypothesis that the increased severity of rotavirus-associated gastroenteritis is not due to its age distribution but to features intrinsic to the virus itself.
Patients and methods
The study was performed in eight hospitals located in six Italian towns (Naples, Turin, Vicenza, Palermo, Parma and Milan). The study protocol was agreed upon by paediatricians, microbiologists and a statistician at a specific investigators’ meeting. All children aged between 1 month and 4 years admitted to hospital for at least 48 h because of acute diarrhoea were prospectively enrolled over 12 months of observation. Children were excluded if they had received antibiotics in the previous seven days or if they had conditions related to immune deficiency. Diarrhoea was defined as the passage of three or more liquid or semi-liquid stools per day. The duration of diarrhoea was the time from the first to the last output of abnormal stools preceding two normal stools. Hospital admission was decided by the medical staff of each hospital, which did not include the investigators taking part in the study unless they were on duty in the emergency room.
Upon admission to hospital, the following information was collected on a specific form: age, sex, body weight, duration of diarrhoea prior to admission and the main reason for admission. Clinical assessment was initially performed in the emergency room and, subsequently, every day during hospitalisation. The following parameters were recorded: body weight, number of stools per day, stool consistency (defined as “solid,” “loose” or “liquid” and were scored accordingly), vomiting episodes and body temperature. Hydration status was assessed daily using the Gorelick score [14]. Diarrhoea was treated with oral and/or parenteral rehydration. Specific drugs were not used unless considered necessary in individual cases by the medical staff.
The severity of diarrhoea was established using a validated score devised by Ruuska and Vesikari [26], in which points are assigned to: length of hospital stay, maximal frequency and duration of diarrhoeal stools, maximal frequency and duration of vomiting, maximal body temperature and severity of dehydration. The maximal score is 20. Gastroenteritis was considered mild at a score between 0–7, moderate between 8–14 and severe between 15–20. The score for each child enrolled in the study was estimated upon discharge from hospital. To compare the disease severity in relation to age, the children were divided into four age groups: 0–6 months, 7–12 months, 13–24 months and >24 months.
Stool specimens were collected from all children and tested for rotavirus with an enzyme immunoassay with anti-rotavirus antibody (Rotazyme test, Abbott Laboratories, Rome, Italy).
All positive samples were frozen and shipped for confirmation to a reference laboratory, where they were analysed for G type by enzyme immunoassay (EIA) using type 1-, 2-, 3- and 4-specific neutralising monoclonal antibodies reactive with viral protein VP7 [9]. The strains that were not typeable by EIA were analysed by a semi-nested polymerase chain reactions (PCR) using primers specific for G1–G4 and G9 types [15].
The samples were also examined for Salmonella, Shigella, Yersinia and Campylobacter, but very few proved to be positive. We therefore evaluated the severity of diarrhoea in rotavirus-positive and in rotavirus-negative children rather than according to specific non-rotavirus pathogens.
The parents of eligible children were informed of the study design and were asked to sign a specific consent form. Children whose parents did not sign the consent form were included in the epidemiologic analysis but not in the clinical evaluation analysis.
Statistical analysis
We used the BMDP statistical software (Biomedical Computer Programs, Berkeley, University of California Press, Los Angeles, CA, USA) for statistical analyses. Mean values for continuous variables were compared using the t-test or the Mann-Whitney test. Differences between proportions were assessed by the chi-square test or by Fisher’s exact test. The adopted level of significance was <0.05.
Results
A total of 911 children (480 males; mean age 17.9±13.3 months, range 1–48 months) with acute diarrhoea were enrolled in the study.
The distribution of children admitted for diarrhoea showed an evident seasonal pattern, with a winter outbreak that peaked in March with 16% of all yearly admissions. The magnitude of the total winter peak of diarrhoea-associated disease accounted for an average of 40% of all diarrhoea-associated hospitalisations.
Rotavirus infection was significantly more frequent during the winter period: overall 71% (265/372) of all observed cases of rotavirus diarrhoea occurred between January and April.
Admission of children negative for rotavirus was prevalent during the fall (46% of all cases of non-rotaviral diarrhoea), with a significantly increased prevalence compared to rotavirus diarrhoea in the summer and autumn (81% and 73% of cases, respectively).
Rotavirus was detected in 381 of 911 (42%) children. Results of bacterial cultures were available for 46.5% (424/911) of the enrolled children. A total of 29 (6.8%) Salmonella, 6 (1.4%) Campylobacter and 1 Shigella infections were detected and the majority of stool cultures yielded negative results.
As shown in Table 1, there was no difference between rotavirus-positive and rotavirus-negative children in terms of sex, body weight or length.
The mean age was also similar. In addition, the duration of diarrhoea, i.e. the time from the onset of diarrhoea to the first observation in the emergency room, was similar, as was the degree of dehydration at the first clinical evaluation.
The incidence of rotavirus and non-rotavirus gastroenteritis was similar in children from 1–24 months (Fig. 1). Rotavirus-negative diarrhoea was more frequent than rotavirus-positive diarrhoea in children older than 24 months (66% vs. 34%, p<0.001). Disease severity was evaluated in children for whom complete clinical data were available. Children with confounding conditions were also excluded. A total of 123 children were excluded because of incomplete data and permission to use personal data was not obtained for 64 children. In addition, 135 children were excluded because they had other underlying conditions or concomitant acute infections (generally of the respiratory tract). Overall, the clinical data of 589 children were analysed. Their aetiological pattern and age distribution reflected that of the whole population.
Table 2 shows the individual items that constitute the diarrhoea severity scores together with the total scores of children with and without rotavirus.
The mean total duration of diarrhoea was 5.6±0.1 days but was significantly longer in rotavirus-positive children than in rotavirus-negative children. In parallel, hospital stays were longer in rotavirus-positive children than in rotavirus-negative children.
Children with rotavirus diarrhoea had a more severe clinical course than rotavirus-negative children. The degree of dehydration was greater in rotavirus-positive than in rotavirus-negative children. There was no difference between the two groups with respect to the degree of dehydration at the first evaluation in the emergency room (Table 1). However, the day after admission, 26% of children with rotaviral gastroenteritis had moderate/severe dehydration versus 11% of children with non-rotavirus gastroenteritis (p<0.05). This trend persisted to the second day of hospitalisation (13% versus 5%; p<0.001). In addition, although the incidence of vomiting was similar, the number of episodes of vomiting was significantly higher in rotavirus diarrhoea patients, with vomiting becoming a more frequent feature the day after admission (data not shown). As a result, parenteral rehydration was more frequent, and lasted longer, in children with rotaviral gastroenteritis (74/222, 33%- vs. 93/367, 25%-; p<0.05 and, respectively, 1.1±0.1 days versus 0.7±0.1 days; p<0.001). Other parameters included in the severity score (body temperature and maximal frequency of diarrhoeal stools) showed an increased incidence, intensity and/or duration in rotavirus gastroenteritis. Overall, as judged by the score, gastroenteritis was more severe in children with rotaviral diarrhoea than in children affected by other or not detected enteric pathogens (Table 2).
Fifty-six children (9.5%) had severe acute gastroenteritis (i.e. a score ≥15) and most were rotavirus-positive (36/56). Thus, severe gastroenteritis was significantly more common in the rotaviral gastroenteritis group (36/222, 15.7%) than in the non-rotaviral gastroenteritis group (20/367, 5.4%; p<0.0001). The incidence of protracted (>15 days) diarrhoea was similar in rotavirus-positive and rotavirus-negative children (7/222 vs. 8/367).
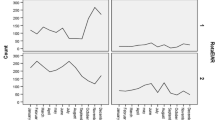
To test the hypothesis that the severity of gastroenteritis was related to aetiology rather than age, we evaluated the severity score in the four prospectively established age groups. The main severity parameters, i.e. total duration of diarrhoea, length of hospital stay, and the need for and duration of parenteral rehydration, were not associated with age. The only age-related specific feature was a lower score in children between 1 and 6 months of age compared with older children (Fig. 2).
To verify the concept that rotavirus diarrhoea is intrinsically more severe, we analysed the severity parameters in rotavirus-positive and rotavirus-negative children, matched for age. The score was consistently higher in rotavirus-positive than in rotavirus-negative children in all age groups (Fig. 3).
Rotavirus serotypes
Of the 381 rotavirus-positive samples, serotyping was carried out for 344 (90%) cases. One-hundred-and-thirteen (30%) of the 381 samples could not be serotyped by EIA and were genotyped. Most strains were G1 (168 by EIA and 33 by PCR) or G4 (91 by EIA and 12 by PCR). The G9 strain accounted for 9% (30 by PCR); G2 and G3 were less frequent (2 and 5, respectively). Double G1-G4 serotypes were detected in 1% of cases (Table 3). Thirty-seven strains were non-typeable by either method. There was no association between specific rotavirus serotypes and the severity of diarrhoea (data not shown).
Discussion
Rotavirus is responsible for a substantial, yet underestimated, number of hospitalisations of children with acute gastroenteritis [17]. The incidence of rotavirus diarrhoea requiring hospital admission in children presenting at the emergency department was 40%, which is similar to that reported in other European countries [11, 18, 20, 21], but lower than the 70% rate found in Canada [24].
Our prospective study provides comparative data on the severity of rotavirus-positive and rotavirus-negative gastroenteritis in children in a hospital setting and shows that aetiology and not age is the major determinant of severity in childhood gastroenteritis. This finding is important in view of the availability of new vaccines against rotavirus in order to evaluate immunisation programmes.
It is commonly considered that gastroenteritis is more severe in the first two years of age and this age corresponds to the peak of rotavirus infection. Rotavirus, in turn, is regarded as a more aggressive enteric pathogen than other agents of childhood diarrhoea [16]. Velazquez et al. [30] showed that the incidence of any type of rotavirus infection is generally highest among infants 6–14 months old and decreases in children 21–23 months old. They also reported a high incidence of reinfection. However, subsequent infections are much less severe and symptoms are negligible in most cases’ symptoms [29]. Thus, severe rotavirus-induced diarrhoea was strongly related to the first infection. In fact, 85% of the risk of moderate to severe rotavirus-associated diarrhoea occurred in the first 8 months of life, 15% occurred between ages 9 and 17 months and there was no risk after the age of 18 months [30]. Because of the peculiar age distribution of rotavirus, we hypothesised that the increased severity of rotavirus could be the result of a more vulnerable (because of the young age) preferred target. On the other hand, given the high frequency of rotavirus infection, the increased severity of diarrhoea in infants and younger children could result from a high exposure to rotavirus. The only age-related specific pattern of diarrhoea severity in our study was a decreased severity in infants younger than 6 months old. In contrast, the severity of diarrhoea was clearly associated with rotavirus aetiology. Within each age group, the total severity score was significantly higher in rotavirus-positive than in rotavirus-negative children. The episodes of vomiting were more frequent and lasted longer, and the number of liquid stools was greater in rotavirus-positive children. Given the more frequent association between dehydration and vomiting than between dehydration and stool number, vomiting played a major role in inducing dehydration.
Finally, we did not find an association between clinical severity and specific viral serotypes. An increased severity has been reported in children infected with G4 type rotavirus [3], but this was not confirmed in other studies [2, 33]. Interestingly, 9% of strains were G9, a serotype that is increasingly observed worldwide [7], with potentially important consequences for vaccine efficacy [28].
The increased severity of rotavirus infection is certainly associated with increased costs for hospital and medical care. In our study, the total duration of symptoms was higher in rotavirus-positive than in rotavirus-negative children and was associated with a longer hospitalisation.
A trend towards increased duration of rotavirus diarrhoea has been reported also in an outpatient setting and was associated with increased costs [1]. It was estimated that the cost of a single episode of diarrhoea requiring an office visit was US$289, irrespective of aetiology, but was US$325 for rotavirus-induced diarrhoea. Our data suggest a similar pattern for hospitalised children who need more intensive and longer medical care.
In conclusion, it is now clear that the intrinsic features of rotavirus rather than age are associated with severe gastroenteritis in children. Our data show that rotavirus is an exceedingly frequent and aggressive pathogen that has substantial clinical and economic consequences. Consequently, efforts aimed at producing a vaccine are well warranted.
Abbreviations
- EIA:
-
enzyme immunoassay
- PCR:
-
polymerase chain reaction
References
Avendano P, Matson DO, Long J, Whitney S, Matson CC, Pickering LK (1993) Costs associated with office visits for diarrhea in infants and toddlers. Pediatric Infect Dis J 12(11):897–902
Bern C, Unicomb L, Gentsch JR, Banul N, Yunus M, Sack RB, Glass RI (1992) Rotavirus diarrhea in Bangladeshi children: correlation of disease severity with serotypes. J Clin Microbiol 30(12):3234–3238
Cascio A, Vizzi E, Alaimo C, Arista S (2001) Rotavirus gastroenteritis in Italian children: can severity of symptoms be related to the infecting virus? Clin Infect Dis 32(8):1126–1132
Centers for Disease Control and Prevention (CDC) (1998) Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 48(RR-2):1–20
Centers for Disease Control and Prevention (CDC) (1999) Intussusceptions among recipients of rotavirus vaccine—United States, 1998–1999. MMWR Morb Mortal Wkly Rep 48(27):577–581
Centers for Disease Control and Prevention (CDC) (1999) Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep 48(43):1007
Clark HF, Lawley DA, Schaffer A, Patacsil JM, Marcello AE, Glass RI, Jain V, Gentsch J (2004) Assessment of the epidemic potential of a new strain of rotavirus associated with the novel G9 the epidemic potential of a new strain of rotavirus associated with the novel G9 serotype which caused an outbreak in the United States for the first time in the 1995–1996 season. J Clin Microbiol 42(4):1434–1438
Cohen MB (1991) Etiology and mechanisms of acute infectious diarrhea in infants in the United States. J Pediatric 118(4 Pt 2):S34–S39
Coulson BS, Unicomb LE, Pitson GA, Bishop RF (1987) Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol 25(3):509–515
Ehlken B, Laubereau B, Karmaus W, Petersen G, Rohwedder A, Forster J; RoMoD Study Group (2002) Prospective population-based study on rotavirus disease in Germany. Acta Paediatric 91(7):769–775
Fruhwirth M, Heininger U, Ehlken B, Petersen G, Laubereau B, Moll-Schuler I, Mutz I, Forster J (2001) International variation in disease burden of rotavirus gastroenteritis in children with community- and nosocomially acquired infection. Pediatric Infect Dis J 20(8):784–791
Fruhwirth M, Karmaus W, Moll-Schuler I, Brosl S, Mutz I (2001) A prospective evaluation of community acquired gastroenteritis in paediatric practices: impact and disease burden of rotavirus infection. Arch Dis Child 84(5):393–397
Gonzales-Adriano SR, Valdes-Garza HE, Garcia-Valdes LC (1988) Hidratacion oral versus hidratacion endovenosa en pacientes con diarrea aguda. Bol Med Hosp Infant Mex 45(3):165–172
Gorelick MH, Shaw KN, Murphy KO (1997) Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics 99(5):e6
Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, Fang ZY (1990) Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol 28(2):276–282
Guarino A, Albano F (2004) Viral diarrhea. In: Guandalini S (ed) Textbook of pediatric gastroenterology and nutrition. Taylor and Francis, London, UK, pp 127–144
Hsu VP, Staat MA, Roberts N, Thieman C, Bernstein DI, Bresee J, Glass RI, Parashar UD (2005) Use of active surveillance to validate International Classification of Diseases code estimates of rotavirus hospitalizations in children. Pediatrics 115(1):78–82
Johansen K, Bennet R, Bondesson K, Eriksson M, Hedlund KO, De Verdier Klingenberg K, Uhnoo I, Svensson L (1999) Incidence and estimates of the disease burden of rotavirus in Sweden. Acta Paediatric Suppl 88(426):20–23
Kapikian AZ (1996) Overview of viral gastroenteritis. Arch Virol Suppl 12:7–19
Matson DO, Estes MK (1990) Impact of rotavirus infection at a large pediatric hospital. J Infect Dis 162(3):598–604
Mrukowicz JZ, Krobicka B, Duplaga M, Kowalska-Duplaga K, Domanski J, Szajewska H, Kantecki M, Iwanczak F, Pytrus T (1999) Epidemiology and impact of rotavirus diarrhoea in Poland. Acta Paediatric Suppl 88(426):53–60
Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, Zanardi LR, Setia S, Fair E, LeBaron CW, Wharton M, Livengood JR; Rotavirus Intussusception Investigation Team (2001) Intussusceptions among infants given an oral rotavirus vaccine. N Engl J Med 344(8):564–572
Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI (2003) Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 9(5):565–572
Rivest P, Proulx M, Lonergan G, Lebel MH, Bedard L (2004) Hospitalizations for gastroenteritis: the role of rotavirus. Vaccine 22(15–16):2013–2017
Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, Lopez P, Macias-Parra M, Ortega-Barria E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavia-Ruz N, Salmeron J, Ruttimann R, Tinoco JC, Rubio P, Nunez E, Guerrero ML, Yarzabal JP, Damaso S, Tornieporth N, Saez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O’Ryan M; Human Rotavirus Vaccine Study Group (2006) Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 354(1):11–22
Ruuska T, Vesikari T (1990) Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 22(3):259–267
Ruuska T, Vesikari T (1991) A prospective study of acute diarrhea in Finnish children from birth to 2.5 years of age: clinical severity, etiology and risk factors. Acta Paediatric Scand 80(5):500–507
Santos N, Hoshino Y (2005) Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 15(1):29–56
Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM (1996) Rotavirus infection in infants as protection against subsequent infections. N Engl J Med 335(14):1022–1028
Velazquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, Glass RI, Pickering LK, Ruiz-Palacios GM (2000) Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis 182(6):1602–1609
Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O’Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM; Rotavirus Efficacy and Safety Trial (REST) Study Team (2006) Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 354(1):23–33
Widdowson MA, Bresee JS, Gentsch JR, Glass RI (2005) Rotavirus disease and its prevention. Curr Opin Gastroenterol 21(1):26–31
Yolken RH, Wyatt RG, Zissis G, Brandt CD, Rodriguez WJ, Kim HW, Parrott RH, Urrutia JJ, Mata L, Greenberg HB, Kapikian AZ, Chanock RM (1978) Epidemiology of human rotavirus Types 1 and 2 as studied by enzyme-linked immunosorbent assay. N Engl J Med 299(21):1156–1161
Acknowledgement
This work was financially supported by a grant from the Italian Ministry of Health, 4th AIDS Research Project, Program 50 D.28.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albano, F., Bruzzese, E., Bella, A. et al. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr 166, 241–247 (2007). https://doi.org/10.1007/s00431-006-0237-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0237-6